Microgels of N-Isopropylacrylamide Copolymerized with an Amphiphilic Acid for the Delivery of Doxorubicin
- PMID: 39727564
- PMCID: PMC11675903
- DOI: 10.3390/gels10120806
Microgels of N-Isopropylacrylamide Copolymerized with an Amphiphilic Acid for the Delivery of Doxorubicin
Abstract
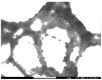
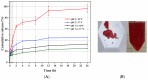
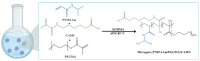
This study aims to design microgels that are thermo- and pH-sensitive for controlled doxorubicin (Dox) release in response to tumor microenvironment changes. N-isopropylacrylamide (NIPAAm) is widely used for thermoresponsive tumor-targeted drug delivery systems for the release of therapeutic payloads in response to temperature changes. Herein, a NIPAAm microgel (MP) that is responsive to temperature and pH was designed for the smart delivery of Dox. MP was made from NIPAAm, and polyethylene glycol methyl ether methacrylate (PEGMA) was copolymerized with 5%, 10%, or 15% mol of methacryloylamido hexanoic acid, (CAM5) an amphiphilic acid. We characterized the microgels using FTIR-ATR, DLS, and FESEM. The MP 10% CAM5 exhibited a particle size of 268 nm, with a transition temperature of 44 °C. MP had a drug loading capacity of 13% and entrapment efficiency of 87%. Nearly 100% of the Dox was released at pH 5 and 42 °C, compared to 30% at pH 7.4 and 37 °C. MP 10% CAM5 showed cytocompatibility in HeLa cells using the MTT assay. However, the cell viability assay showed that dox-MP was twice as effective as free Dox. Specifically, 3 μg/mL of free Dox resulted in 74% cell viability, while the same doses of Dox in NP reduced it to 35%. These results are promising for the future tumor-targeted delivery of antineoplastic-drugs, as they may reduce the side effects of Dox.
Keywords: doxorubicin; microgels; poly N-isopropylacrylamide; stimuli responsive materials.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





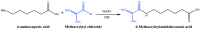
Similar articles
-
A new style for synthesis of thermo-responsive Fe3O4/poly (methylmethacrylate-b-N-isopropylacrylamide-b-acrylic acid) magnetic composite nanosphere and theranostic applications.J Biomater Sci Polym Ed. 2017 Dec;28(17):1985-2005. doi: 10.1080/09205063.2017.1364459. Epub 2017 Aug 18. J Biomater Sci Polym Ed. 2017. PMID: 28783443
-
Encapsulation of Thermo-responsive Gel in pH-sensitive Polymersomes as Dual-Responsive Smart carriers for Controlled Release of Doxorubicin.J Control Release. 2018 Oct 28;288:45-61. doi: 10.1016/j.jconrel.2018.08.039. Epub 2018 Aug 30. J Control Release. 2018. PMID: 30171978
-
Folic acid conjugated palygorskite/Au hybrid microgels: Temperature, pH and light triple-responsive and its application in drug delivery.Colloids Surf B Biointerfaces. 2023 Sep;229:113432. doi: 10.1016/j.colsurfb.2023.113432. Epub 2023 Jun 28. Colloids Surf B Biointerfaces. 2023. PMID: 37422992
-
Temperature-sensitive poly(vinyl alcohol)/poly(methacrylate-co-N-isopropyl acrylamide) microgels for doxorubicin delivery.Biomacromolecules. 2009 Jun 8;10(6):1589-96. doi: 10.1021/bm900185u. Biomacromolecules. 2009. PMID: 19425550
-
Controlled release of doxorubicin from pH-responsive microgels.Acta Biomater. 2013 Mar;9(3):5438-46. doi: 10.1016/j.actbio.2012.09.019. Epub 2012 Sep 25. Acta Biomater. 2013. PMID: 23022545 Free PMC article.
Cited by
-
Hydrogels and Microgels: Driving Revolutionary Innovations in Targeted Drug Delivery, Strengthening Infection Management, and Advancing Tissue Repair and Regeneration.Gels. 2025 Mar 3;11(3):179. doi: 10.3390/gels11030179. Gels. 2025. PMID: 40136884 Free PMC article. Review.
References
-
- Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Pt 1Cancer Res. 1986;46:6387–6392. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

