Ketoprofen Associated with Hyaluronic Acid Hydrogel for Temporomandibular Disorder Treatment: An In Vitro Study
- PMID: 39727570
- PMCID: PMC11675910
- DOI: 10.3390/gels10120811
Ketoprofen Associated with Hyaluronic Acid Hydrogel for Temporomandibular Disorder Treatment: An In Vitro Study
Abstract
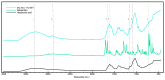
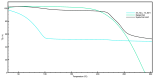
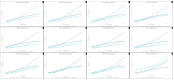
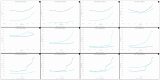
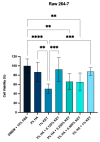
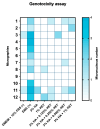
Temporomandibular disorders (TMD) are a public health problem that affects around 12% of the global population. The treatment is based on analgesics, non-steroidal anti-inflammatory, corticosteroids, anticonvulsants, or arthrocentesis associated with hyaluronic acid-based viscosupplementation. However, the use of hyaluronic acid alone in viscosupplementation does not seem to be enough to regulate the intra-articular inflammatory process. So, we propose to develop and evaluate the physicochemical and biological properties in vitro of hyaluronic acid hydrogels (HA) associated with ketoprofen (KET) as a new therapeutic treatment for TMD. The hydrogels were synthesized with 3% HA and 0.125, 0.250, 0.500, or 1% KET. Physicochemical analyses of Attenuated Total reflectance-Fourier transform infrared spectroscopy (FTIR), Thermogravimetry (TGA), Rheology by Frequency, Amplitude sweeps, temperature ramp, and scanning electron microscopy (SEM) were performed with or without sterilization and cycled. Cytocompatibility and genotoxicity (micronucleus assay) were performed in mouse macrophages (RAW 264-7) for 24 h. Results: FTIR spectrum showed characteristic absorptions of HA and KET. In the TGA, two mass loss peaks were observed, the first representing the water evaporation at 30 and 100 °C, and the second peaks between 200 and 300 °C, indicating the degradation of HA and KET. Rheology tests in the oscillatory regime classified the hydrogels as non-Newtonian fluids, time-dependent, and thixotropic. Mouse macrophages (RAW 264-7) presented viability of 83.6% for HA, 50.7% for KET, and 92.4%, 66.1%, 65.3%, and 87.7% for hydrogels, in addition to the absence of genotoxicity. Conclusions: Hyaluronic acid associated with ketoprofen shows satisfactory physicochemical and biological properties for use as viscosupplementation. As a limiting point of this study, further research is needed to evaluate the pharmacodynamic, toxicological, and pharmacokinetic characteristics of a complete organism.
Keywords: non-steroidal anti-inflammatory agents 1; osteoarthritis 3; polysaccharides 2.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Functional hyaluronic acid hydrogels prepared by a novel method.Mater Sci Eng C Mater Biol Appl. 2014 Dec;45:573-7. doi: 10.1016/j.msec.2014.10.001. Epub 2014 Oct 2. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 25491866
-
Evaluation of Arthrocentesis with hyaluronic acid injections for management of temporomandibular disorders: a systematic review and case series.J Biol Regul Homeost Agents. 2021 Mar-Apr;35(2 Suppl. 1):21-35. doi: 10.23812/21-2supp1-3. J Biol Regul Homeost Agents. 2021. PMID: 34281300
-
Combination of hyaluronic acid and PLGA particles as hybrid systems for viscosupplementation in osteoarthritis.Int J Pharm. 2019 Mar 25;559:13-22. doi: 10.1016/j.ijpharm.2019.01.017. Epub 2019 Jan 18. Int J Pharm. 2019. PMID: 30664999
-
Hyaluronic Acid/Parecoxib-Loaded PLGA Microspheres for Therapy of Temporomandibular Disorders.Curr Drug Deliv. 2021;18(2):234-245. doi: 10.2174/1567201817999200817151048. Curr Drug Deliv. 2021. PMID: 32811397
-
Efficacy of viscosupplementation with hyaluronic acid in temporomandibular disorders: A systematic review.J Craniomaxillofac Surg. 2018 Nov;46(11):1943-1952. doi: 10.1016/j.jcms.2018.08.007. Epub 2018 Aug 27. J Craniomaxillofac Surg. 2018. PMID: 30249483
Cited by
-
Dexketoprofen-Loaded Alginate-Grafted Poly(N-vinylcaprolactam)-Based Hydrogel for Wound Healing.Int J Mol Sci. 2025 Mar 26;26(7):3051. doi: 10.3390/ijms26073051. Int J Mol Sci. 2025. PMID: 40243670 Free PMC article.
-
Study on the Combined Application of Occlusal Splint and Intra-Articular Injection of Hyaluronic Acid in the Treatment of Non-Reducible Anterior Disc Displacement of the Temporomandibular Joint.Ther Clin Risk Manag. 2025 Apr 23;21:523-532. doi: 10.2147/TCRM.S518989. eCollection 2025. Ther Clin Risk Manag. 2025. PMID: 40297063 Free PMC article.
References
-
- Cardoneanu A., Macovei L.A., Burlui A.M., Mihai I.R., Bratoiu I., Rezus I.I., Richter P., Tamba B.-I., Rezus E. Temporomandibular Joint Osteoarthritis: Pathogenic Mechanisms Involving the Cartilage and Subchondral Bone, and Potential Therapeutic Strategies for Joint Regeneration. Int. J. Mol. Sci. 2022;24:171. doi: 10.3390/ijms24010171. - DOI - PMC - PubMed
Grants and funding
- 2023/05850-4./Fundação de Amparo à Pesquisa do Estado de São Paulo
- 88887.891487/2023-00/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- 88887.829787/2023-00/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- 88887.971513/2024-00/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
LinkOut - more resources
Full Text Sources
Research Materials

