Development and Characterization of Dual-Loaded Niosomal Ion-Sensitive In Situ Gel for Ocular Delivery
- PMID: 39727573
- PMCID: PMC11675977
- DOI: 10.3390/gels10120816
Development and Characterization of Dual-Loaded Niosomal Ion-Sensitive In Situ Gel for Ocular Delivery
Abstract
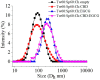
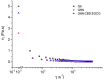
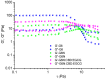
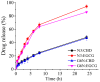
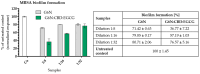
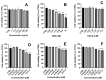
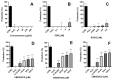
The study investigates the development and characterization of dual-loaded niosomes incorporated into ion-sensitive in situ gel as a potential drug delivery platform for ophthalmic application. Cannabidiol (CBD) and epigallocatechin-3-gallate (EGCG) simultaneously loaded niosomes were prepared via the thin film hydration (TFH) method followed by pulsatile sonication and were subjected to comprehensive physicochemical evaluation. The optimal composition was included in a gellan gum-based in situ gel, and the antimicrobial activity, in vitro toxicity in a suitable corneal epithelial model (HaCaT cell line), and antioxidant potential of the hybrid system were further assessed. Dual-loaded niosomes based on Span 60, Tween 60, and cholesterol (3.5:3.5:3 mol/mol) were characterized by appropriate size (250 nm), high entrapment efficiency values for both compounds (85% for CBD and 50% for EGCG) and sustained release profiles. The developed hybrid in situ gel exhibited suitable rheological characteristics to enhance the residence time on the ocular surface. The conducted microbiological studies reveal superior inhibition of methicillin-resistant Staphylococcus aureus (MRSA) adhesion by means of the niosomal in situ gel compared to the blank gel and untreated control. Regarding the antioxidant potential, the dual loading of CBD and EGCG in niosomes enhances their protective properties, and the inclusion of niosomes in gel form preserves these effects. The obtained outcomes indicate the developed niosomal in situ gel as a promising drug delivery platform in ophthalmology.
Keywords: HaCaT cells; cannabidiol; epigallocatechin-3-gallate; gellan gum; ocular delivery; rheological studies; sol gel transition.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Formulation and Evaluation of Hybrid Niosomal In Situ Gel for Intravesical Co-Delivery of Curcumin and Gentamicin Sulfate.Pharmaceutics. 2022 Mar 30;14(4):747. doi: 10.3390/pharmaceutics14040747. Pharmaceutics. 2022. PMID: 35456581 Free PMC article.
-
Formulation and Evaluation of Niosomal in situ Nasal Gel of a Serotonin Receptor Agonist, Buspirone Hydrochloride for the Brain Delivery via Intranasal Route.Pharm Nanotechnol. 2018;6(1):69-78. doi: 10.2174/2211738506666180130105919. Pharm Nanotechnol. 2018. PMID: 29380709
-
Development and evaluation of doxycycline niosomal thermoresponsive in situ gel for ophthalmic delivery.Int J Pharm. 2020 Dec 15;591:120010. doi: 10.1016/j.ijpharm.2020.120010. Epub 2020 Oct 22. Int J Pharm. 2020. PMID: 33132152
-
Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation.J Pharm Pharmacol. 2019 Aug;71(8):1209-1221. doi: 10.1111/jphp.13106. Epub 2019 May 24. J Pharm Pharmacol. 2019. PMID: 31124593
-
Gellan gum-based in-situ gel formulations for ocular drug delivery: A practical approach.Int J Biol Macromol. 2025 Feb;290:138979. doi: 10.1016/j.ijbiomac.2024.138979. Epub 2024 Dec 19. Int J Biol Macromol. 2025. PMID: 39708866 Review.
Cited by
-
Nanovesicular Drug Delivery Systems for Rare Ocular Diseases: Advances, Challenges, and Future Directions.AAPS PharmSciTech. 2025 Jul 23;26(7):197. doi: 10.1208/s12249-025-03159-8. AAPS PharmSciTech. 2025. PMID: 40702295 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

