Survival Outcomes with Regorafenib and/or Trifluridine/Tipiracil Sequencing to Rechallenge with Third-Line Regimens in Metastatic Colorectal Cancer: A Multicenter Retrospective Real-World Subgroup Comparison from the ReTrITA Study
- PMID: 39727697
- PMCID: PMC11674570
- DOI: 10.3390/curroncol31120574
Survival Outcomes with Regorafenib and/or Trifluridine/Tipiracil Sequencing to Rechallenge with Third-Line Regimens in Metastatic Colorectal Cancer: A Multicenter Retrospective Real-World Subgroup Comparison from the ReTrITA Study
Abstract
Background: There is ongoing discussion around the optimal course of treatment for metastatic colorectal cancer (mCRC) following the second line. Trifluridine/tipiracil (T) and regorafenib (R) have been the mainstay of therapy in this situation, as they both increased overall survival (OS) in comparison to a placebo. Despite the paucity of evidence, therapy rechallenge is also recognized as an option for practical use. In the third-line scenario of mCRC, we planned to investigate the survival outcomes using (T) and (R), both with and without prior rechallenge treatment.
Materials and methods: Between 2012 and 2023, we examined the medical records of 1156 patients with refractory mCRC who were enrolled in the multicenter retrospective ReTrITA study. We then separated the patients into two cohorts based on the rechallenge therapy that was given before regorafenib and/or trifluridine/tipiracil at 17 Italian centres.
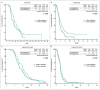
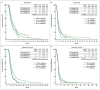
Results: A total of 981 patients underwent T and/or R therapy, while 175 patients had therapy rechallenge before T and/or R. The median overall survival (mOS) for patients treated with T/R and R/T sequences in the rechallenge therapy cohort was 14.5 months and 17.6 months, respectively (p = 0.1955). A statistically significant survival benefit was observed in patients who received monotheraphy with R (mOS: 6 months) compared to the T group (mOS: 4.2 months) (p = 0.0332). In the same cohort, a median progression-free survival (mPFS) benefit was demonstrated in favour of the R/T group (11.3 months) vs. 9 months of the reverse sequence (p = 0.4004). In the no-rechallenge cohort, the mOS was statistically longer in the R/T sequence than in the T/R sequence (16.2 months vs. 12.3 months, respectively; p = 0.0014). In terms of the mPFS, we saw the same significant result for the adoption of R/T treatment (11.5 months vs. 8.4 months, respectively; p < 0.0001). The two monotherapy groups did not reveal any significant differences.
Conclusions: This study suggests that rechallenge therapy may improve survival rates in the third-line treatment of mCRC, particularly if it is administered before sequential R/T treatment. This could allow for the extension of mCRC treatment choices until prospective studies are finished or randomised trials are performed.
Keywords: metastatic colorectal cancer; real-world study; rechallenge therapy; regorafenib; third-line therapy; trifluridine/tipiracil.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Colon Cancer Version 5.2024—August 22, 2024. [(accessed on 18 September 2024)]. Available online: www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
-
- Van Cutsem E., Nordlinger B., Adam R., Köhne C.-H., Pozzo C., Poston G., Ychou M., Rougier P., European Colorectal Metastases Treatment Group Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. - DOI - PubMed
-
- Kemeny N. Management of liver metastases from colorectal cancer. Oncology. 2006;20:1161–1186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

