Dehydrated Human Amnion-Chorion Membrane as a Bioactive Scaffold for Dental Pulp Tissue Regeneration
- PMID: 39727775
- PMCID: PMC11727341
- DOI: 10.3390/biomimetics9120771
Dehydrated Human Amnion-Chorion Membrane as a Bioactive Scaffold for Dental Pulp Tissue Regeneration
Abstract
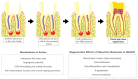
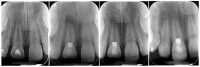
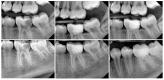
The dehydrated human amnion-chorion membranes (dHACMs) derived from the human placenta have emerged as a promising biomaterial for dental pulp regeneration owing to their unique biological and structural properties. The purpose of this review is to explore the potentials of dHACMs in dental pulp tissue engineering, focusing on their ability to promote cellular proliferation, differentiation, angiogenesis, and neurogenesis. dHACMs are rich in extracellular matrix proteins and growth factors such as TGF-β1, FGF2, and VEGF. They also exhibit significant anti-inflammatory and antimicrobial properties, creating an optimal environment for dental pulp regeneration. The applications of dHACMs in regenerative endodontic procedures are discussed, highlighting their ability to support the formation of dentin and well-vascularized pulp-like tissue. This review demonstrates that dHACMs hold significant potential for enhancing the success of pulp regeneration and offer a biologically based approach to preserve tooth vitality and improve tooth survival. Future research is expected to focus on conducting long-term clinical studies to establish their efficacy and safety.
Keywords: amnion–chorion membranes; bioactive scaffolds; biomaterials; dental pulp regeneration; regenerative endodontics.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

