Immune-Related Adverse Events in a Patient Treated with Pembrolizumab: A Case Report from the Point of View of a Geriatrician
- PMID: 39727819
- PMCID: PMC11728412
- DOI: 10.3390/geriatrics9060160
Immune-Related Adverse Events in a Patient Treated with Pembrolizumab: A Case Report from the Point of View of a Geriatrician
Abstract
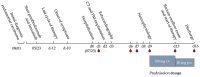
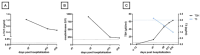
We report the case of a 78-year-old female patient who received palliative immunotherapy with pembrolizumab and lenvatinib as a treatment of pulmonary and osseous metastatic endometrial carcinoma. Under this therapy, the patient developed dysphagia, thyroiditis with hypothyroidism, myositis, and myocarditis, which required, due to third-degree AV block, the installation of a pacemaker. The patient received high-dose cortisone therapy, a thyroid hormone substitution, and pyridostigmine for symptom control. With this therapy, we saw a significant but not complete regression of symptoms. Ultimately, we could discharge the patient home for an outpatient treatment. The case report is followed by a discussion of the management of immune-related adverse events (irAEs) during pembrolizumab therapy from a geriatric perspective. Elderly patients on pembrolizumab therapy require close monitoring for irAEs, which can present atypically or without symptoms and may be fatal. Non-invasive diagnostics and minimizing hospital stays are essential to preserve the fitness of this vulnerable population.
Keywords: geriatrics; immune checkpoint inhibitor; immune myocarditis; immune-related adverse events; pembrolizumab; third-degree atrioventricular block.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Pembrolizumab-induced myocarditis with complete atrioventricular block and concomitant myositis in a metastatic bladder cancer patient: a case report and review of the literature.J Med Case Rep. 2024 Feb 22;18(1):107. doi: 10.1186/s13256-024-04397-3. J Med Case Rep. 2024. PMID: 38383436 Free PMC article. Review.
-
Pembrolizumab-Induced Myasthenia Gravis With Myocarditis in the Setting of Metastatic Renal Cell Carcinoma.Cureus. 2024 Aug 31;16(8):e68318. doi: 10.7759/cureus.68318. eCollection 2024 Aug. Cureus. 2024. PMID: 39350808 Free PMC article.
-
A Fatal Case of Myocarditis Following Myositis Induced by Pembrolizumab Treatment for Metastatic Upper Urinary Tract Urothelial Carcinoma.Int Heart J. 2020 Sep 29;61(5):1070-1074. doi: 10.1536/ihj.20-162. Epub 2020 Sep 12. Int Heart J. 2020. PMID: 32921673
-
Hypothyroidism and hypopituitarism as immune-related adverse events due to lenvatinib plus pembrolizumab therapy in the immediate postoperative period after laparoscopic hepatectomy for liver metastases from gastric cancer: a case report.Surg Case Rep. 2021 Dec 20;7(1):267. doi: 10.1186/s40792-021-01346-w. Surg Case Rep. 2021. PMID: 34928436 Free PMC article.
-
Pembrolizumab-Induced Fatal Myasthenia, Myocarditis, and Myositis in a Patient with Metastatic Melanoma: Autopsy, Histological, and Immunohistochemical Findings-A Case Report and Literature Review.Int J Mol Sci. 2023 Jun 30;24(13):10919. doi: 10.3390/ijms241310919. Int J Mol Sci. 2023. PMID: 37446095 Free PMC article. Review.
References
-
- Arora S., Balasubramaniam S., Zhang W., Zhang L., Sridhara R., Spillman D., Mathai J.P., Scott B., Golding S.J., Coory M., et al. FDA Approval Summary: Pembrolizumab plus Lenvatinib for Endometrial Carcinoma, a Collaborative International Review under Project Orbis. Clin. Cancer Res. 2020;26:5062–5067. doi: 10.1158/1078-0432.CCR-19-3979. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

