Overview of Gas-Generating-Reaction-Based Immunoassays
- PMID: 39727844
- PMCID: PMC11726966
- DOI: 10.3390/bios14120580
Overview of Gas-Generating-Reaction-Based Immunoassays
Abstract
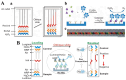
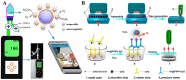
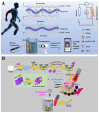
Point-of-care (POC) immunoassays have become convincing alternatives to traditional immunosensing methods for the sensitive and real-time detection of targets. Immunoassays based on gas-generating reactions were recently developed and have been used in various fields due to their advantages, such as rapid measurement, direct reading, simple operation, and low cost. Enzymes or nanoparticles modified with antibodies can effectively catalyze gas-generating reactions and convert immunorecognition events into gas pressure signals, which can be easily recorded by multifunctional portable devices. This article summarizes the advances in gas-generating-reaction-based immunoassays, according to different types of signal output systems, including distance-based readout, pressure differential, visualized detection, and thermal measurement. The review mainly focuses on the role of photothermal materials and the working principle of immunoassays. In addition, the challenges and prospects for the future development of gas-generating-reaction-based immunoassays are briefly discussed.
Keywords: gas generating; immunoassays; nanozymes; point-of-care testing; pressure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Xia N., Liu G., Chen Y., Wu T., Liu L., Yang S., Li Y. Magnetically-assisted electrochemical immunoplatform for simultaneous detection of active and total prostate-specific antigen based on proteolytic reaction and sandwich affinity analysis. Talanta. 2024;270:125534. doi: 10.1016/j.talanta.2023.125534. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

