Nanolabels Prepared by the Entrapment or Self-Assembly of Signaling Molecules for Colorimetric and Fluorescent Immunoassays
- PMID: 39727862
- PMCID: PMC11674709
- DOI: 10.3390/bios14120597
Nanolabels Prepared by the Entrapment or Self-Assembly of Signaling Molecules for Colorimetric and Fluorescent Immunoassays
Abstract
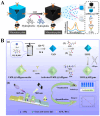
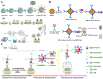
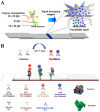
Nanomaterials have attracted significant attention as signal reporters for immunoassays. They can directly generate detectable signals or release a large number of signaling elements for readout. Among various nanolabels, nanomaterials composed of multiple signaling molecules have shown great potential in immunoassays. Generally, signaling molecules can be entrapped in nanocontainers or self-assemble into nanostructures for signal amplification. In this review, we summarize the advances of signaling molecules-entrapped or assembled nanomaterials for colorimetric and fluorescence immunoassays. The nanocontainers cover liposomes, polymers, mesoporous silica, metal-organic frameworks (MOFs), various nanosheets, nanoflowers or nanocages, etc. Signaling molecules mainly refer to visible and/or fluorescent organic dyes. The design and application of immunoassays are emphasized from the perspective of nanocontainers, analytes, and analytical performances. In addition, the future challenges and research trends for the preparation of signaling molecules-entrapped or assembled nanolabels are briefly discussed.
Keywords: immunoassays; nanocontainers; nanomaterials; self-assembly; signaling molecules.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Colorimetric Immunoassays with Boronic Acid-Decorated, Peroxidase-like Metal-Organic Frameworks as the Carriers of Antibodies and Enzymes.Molecules. 2024 Jun 24;29(13):3000. doi: 10.3390/molecules29133000. Molecules. 2024. PMID: 38998952 Free PMC article.
-
Overview of the Design and Application of Dual-Signal Immunoassays.Molecules. 2024 Sep 25;29(19):4551. doi: 10.3390/molecules29194551. Molecules. 2024. PMID: 39407482 Free PMC article. Review.
-
Advancements in Biosensors Based on the Assembles of Small Organic Molecules and Peptides.Biosensors (Basel). 2023 Jul 29;13(8):773. doi: 10.3390/bios13080773. Biosensors (Basel). 2023. PMID: 37622859 Free PMC article. Review.
-
Overview of the Design and Application of Photothermal Immunoassays.Sensors (Basel). 2024 Oct 6;24(19):6458. doi: 10.3390/s24196458. Sensors (Basel). 2024. PMID: 39409498 Free PMC article. Review.
-
State-of-the-art progress of switch fluorescence biosensors based on metal-organic frameworks and nucleic acids.Mikrochim Acta. 2021 Apr 21;188(5):168. doi: 10.1007/s00604-021-04827-9. Mikrochim Acta. 2021. PMID: 33884514 Review.
Cited by
-
Establishment of a Rapid and Convenient Fluoroimmunoassay Platform Using Antibodies Against PDL1 and HER2.Curr Issues Mol Biol. 2025 Jan 17;47(1):62. doi: 10.3390/cimb47010062. Curr Issues Mol Biol. 2025. PMID: 39852177 Free PMC article.
References
-
- Pan R., Li G., Liu S., Zhang X., Liu J., Su Z., Wu Y. Emerging nanolabels-based immunoassays: Principle and applications in food safety. TrAC Trend. Anal. Chem. 2021;145:116462. doi: 10.1016/j.trac.2021.116462. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

