Establishment of Sample-to-Answer Loop-Mediated Isothermal Amplification-Based Nucleic Acid Testing Using the Sampling, Processing, Incubation, Detection and Lateral Flow Immunoassay Platforms
- PMID: 39727874
- PMCID: PMC11674888
- DOI: 10.3390/bios14120609
Establishment of Sample-to-Answer Loop-Mediated Isothermal Amplification-Based Nucleic Acid Testing Using the Sampling, Processing, Incubation, Detection and Lateral Flow Immunoassay Platforms
Abstract
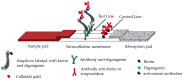
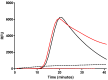
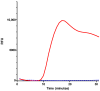
Diagnostics often require specialized equipment and trained personnel in laboratory settings, creating a growing need for point-of-care tests (POCTs). Among the genetic testing methods available, Loop-mediated Isothermal Amplification (LAMP) offers a viable solution for developing genetic POCT due to its compatibility with simplified devices. This study aimed to create a genetic test that integrates all steps from sample processing to analyzing results while minimizing the complexity, handling, equipment, and time required. Several challenges were addressed to achieve this goal: (1) the development of a buffer for bacterial DNA extraction that is compatible with both LAMP and immunochromatographic tests; (2) the adaption of the LAMP protocol for use with the SPID device; and (3) the optimization of the detection protocol for specific test conditions, with a lateral flow immunoassay format selected for its POCT compatibility. Following these developments, the test was validated using Escherichia coli (E. coli) and non-E. coli strains. A portable heating station was also developed to enable amplification without costly equipment. The resulting genetic POCT achieved 100% sensitivity and 85% specificity, with results available in 60 to 75 min. This study demonstrated that our POCT efficiently performs DNA extraction, amplification, and detection for bacterial identification. The test's simplicity and cost-effectiveness will support its implementation in various settings.
Keywords: POCT; genetic detection; sample-to-answer test.
Conflict of interest statement
The SPID device is patented. Hervé Volland is the inventor of this patent (EP3528947).
Figures







References
-
- OMS Point of Care Tests. [(accessed on 3 June 2024)]. Available online: https://www.who.int/teams/sexual-and-reproductive-health-and-research-(s....
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

