Nanopipettes as a Potential Diagnostic Tool for Selective Nanopore Detection of Biomolecules
- PMID: 39727892
- PMCID: PMC11674911
- DOI: 10.3390/bios14120627
Nanopipettes as a Potential Diagnostic Tool for Selective Nanopore Detection of Biomolecules
Abstract
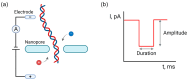
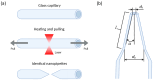
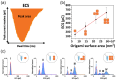
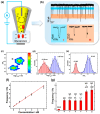
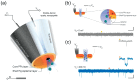
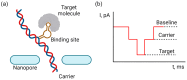
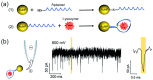
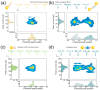
Nanopipettes, as a class of solid-state nanopores, have evolved into universal tools in biomedicine for the detection of biomarkers and different biological analytes. Nanopipette-based methods combine high sensitivity, selectivity, single-molecule resolution, and multifunctionality. The features have significantly expanded interest in their applications for the biomolecular detection, imaging, and molecular diagnostics of real samples. Moreover, the ease of manufacturing nanopipettes, coupled with their compatibility with fluorescence and electrochemical methods, makes them ideal for portable point-of-care diagnostic devices. This review summarized the latest progress in nanopipette-based nanopore technology for the detection of biomarkers, DNA, RNA, proteins, and peptides, in particular β-amyloid or α-synuclein, emphasizing the impact of technology on molecular diagnostics. By addressing key challenges in single-molecule detection and expanding applications in diverse biological areas, nanopipettes are poised to play a transformative role in the future of personalized medicine.
Keywords: molecular diagnostics; nanopipette; nanopore; single molecule; solid-state nanopore.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Ohshiro T. Nanodevices for Biological and Medical Applications: Development of Single-Molecule Electrical Measurement Method. Appl. Sci. 2022;12:1539. doi: 10.3390/app12031539. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

