Development of RT h-CLAT, a Rapid Assessment Method for Skin Sensitizers Using THP-1 Cells as a Biosensor
- PMID: 39727897
- PMCID: PMC11674273
- DOI: 10.3390/bios14120632
Development of RT h-CLAT, a Rapid Assessment Method for Skin Sensitizers Using THP-1 Cells as a Biosensor
Abstract
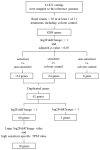
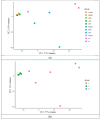
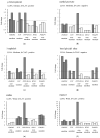
In recent years, in vitro skin sensitization assays have been recommended as animal-free alternatives for the safety assessment of cosmetics and topical drugs, and these methods have been adopted in OECD test guidelines. However, existing assays remain complex and costly. To address this, we recently developed a more efficient, cost-effective, and accurate method for evaluating skin sensitizers by using immune cell-derived THP-1 cells as a biosensor, coupled with an RT-PCR-based assay. In this study, we further refined this method to enable even faster assessment of skin sensitization. By performing comprehensive RNA sequencing (RNA-Seq) analysis, we examined gene expression profiles induced by sensitizers in THP-1 cells to identify potential sensitization markers, ultimately selecting the optimal markers and conditions for evaluation. Our findings indicate that after exposing a test chemical to THP-1 cells for 5 h, measuring the expression levels of the JUN and HMOX1 genes via real-time PCR allows for a reliable assessment of sensitization. A test compound is defined as a sensitizer if either gene shows a more than two-fold increase in its expression compared to the control. Applying this improved method, designated as RT h-CLAT, we evaluated the sensitization potential of 43 chemicals. The results demonstrated higher accuracy compared to the human cell line activation test (h-CLAT) listed in the OECD guidelines, while also reducing the required assessment time from two days to one.
Keywords: HMOX1; JUN; RNA-Seq analysis; alternative methods; biomarker; in vitro skin sensitization test.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Development of an in vitro skin sensitization test using human cell lines; human Cell Line Activation Test (h-CLAT). II. An inter-laboratory study of the h-CLAT.Toxicol In Vitro. 2006 Aug;20(5):774-84. doi: 10.1016/j.tiv.2005.10.014. Epub 2005 Dec 7. Toxicol In Vitro. 2006. PMID: 16337770
-
The relationship between CD86/CD54 expression and THP-1 cell viability in an in vitro skin sensitization test--human cell line activation test (h-CLAT).Cell Biol Toxicol. 2009 Apr;25(2):109-26. doi: 10.1007/s10565-008-9059-9. Epub 2008 Jan 19. Cell Biol Toxicol. 2009. PMID: 18204907
-
Preliminary discovery of novel markers for human cell line activation test (h-CLAT).Toxicol In Vitro. 2021 Aug;74:105154. doi: 10.1016/j.tiv.2021.105154. Epub 2021 Mar 25. Toxicol In Vitro. 2021. PMID: 33774146
-
Non-animal assessment of skin sensitization hazard: Is an integrated testing strategy needed, and if so what should be integrated?J Appl Toxicol. 2018 Jan;38(1):41-50. doi: 10.1002/jat.3479. Epub 2017 May 24. J Appl Toxicol. 2018. PMID: 28543848 Review.
-
State of the art in non-animal approaches for skin sensitization testing: from individual test methods towards testing strategies.Arch Toxicol. 2016 Dec;90(12):2861-2883. doi: 10.1007/s00204-016-1842-4. Epub 2016 Sep 14. Arch Toxicol. 2016. PMID: 27629427 Review.
References
-
- OECD . OECD Guidelines for the Testing of Chemicals. OECD Publishing; Paris, France: 2010. Test No. 429: Skin Sensitisation: Local Lymph Node Assay. Section 4. - DOI
-
- OECD . OECD Guidelines for the Testing of Chemicals. OECD Publishing; Paris, France: 2010. Test No. 442A: Skin Sensitization: Local Lymph Node Assay: DA. Section 4. - DOI
-
- OECD . OECD Guidelines for the Testing of Chemicals. OECD Publishing; Paris, France: 2022. Test No. 406: Skin Sensitisation Guinea Pig Maximisation Test and Bühler Test. Section 4. - DOI
-
- Directive 2003/15/EC, Directive 2003/15/EC of the European parliament and of the council of 27 February 2003 amending council directive 76/768/EEC on the approximation of the laws of the member states relating to cosmetic products. Off. J. Eur. Union. 2003;L66:26–35.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

