Towards Effective Treatment of Glioblastoma: The Role of Combination Therapies and the Potential of Phytotherapy and Micotherapy
- PMID: 39727987
- PMCID: PMC11674107
- DOI: 10.3390/cimb46120859
Towards Effective Treatment of Glioblastoma: The Role of Combination Therapies and the Potential of Phytotherapy and Micotherapy
Abstract
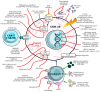
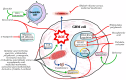
Glioblastoma multiforme (GBM) is one of the most aggressive and difficult-to-treat brain tumors, with a poor prognosis due to its high resistance to conventional therapies. Current treatment options, including surgical resection, radiotherapy, and chemotherapy, have limited effectiveness in improving long-term survival. Despite the emergence of new therapies, monotherapy approaches have not shown significant improvements, highlighting the need for innovative therapeutic strategies. Combination therapies appear to be the most promising solution, as they target multiple molecular pathways involved in GBM progression. One area of growing interest is the incorporation of phytotherapy and micotherapy as complementary treatments, which offer potential benefits due to their anti-tumor, anti-inflammatory, and immunomodulatory properties. This review examines the current challenges in GBM treatment, discusses the potential of combination therapies, and highlights the promising role of phytotherapy and micotherapy as integrative therapeutic options for GBM management.
Keywords: combined therapy; conventional therapy; glioblastoma; innovative therapy; natural adjuvant; resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The power of a novel combined anticancer therapy: challenge and opportunity of micotherapy in the treatment of Glioblastoma Multiforme.Biomed Pharmacother. 2022 Nov;155:113729. doi: 10.1016/j.biopha.2022.113729. Epub 2022 Sep 24. Biomed Pharmacother. 2022. PMID: 36166961
-
Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM).Cancers (Basel). 2023 Apr 1;15(7):2116. doi: 10.3390/cancers15072116. Cancers (Basel). 2023. PMID: 37046777 Free PMC article. Review.
-
Study on the activation of cell death mechanisms: in search of new therapeutic targets in glioblastoma multiforme.Apoptosis. 2023 Aug;28(7-8):1241-1257. doi: 10.1007/s10495-023-01857-x. Epub 2023 May 27. Apoptosis. 2023. PMID: 37244884 Free PMC article.
-
Immune Escape in Glioblastoma Multiforme and the Adaptation of Immunotherapies for Treatment.Front Immunol. 2020 Oct 15;11:582106. doi: 10.3389/fimmu.2020.582106. eCollection 2020. Front Immunol. 2020. PMID: 33178210 Free PMC article. Review.
-
Case Report: Effective management of residual glioblastoma with combined modality therapy.Front Oncol. 2025 Mar 28;15:1451408. doi: 10.3389/fonc.2025.1451408. eCollection 2025. Front Oncol. 2025. PMID: 40224189 Free PMC article.
Cited by
-
Mechanistic insights into the therapeutic potential of β-elemene on glioma and other central nervous system diseases.Med Oncol. 2025 Aug 22;42(10):438. doi: 10.1007/s12032-025-03009-4. Med Oncol. 2025. PMID: 40846815 Review.
-
ATRX, OLIG2, MGMT, and IDH2 in Glioblastoma: Essential Molecular Mechanisms and Therapeutic Significance.Medicina (Kaunas). 2025 Apr 10;61(4):697. doi: 10.3390/medicina61040697. Medicina (Kaunas). 2025. PMID: 40282990 Free PMC article.
-
Simultaneous Multi-Treatment Strategy for Brain Tumor Reduction via Nonlinear Control.Brain Sci. 2025 Feb 17;15(2):207. doi: 10.3390/brainsci15020207. Brain Sci. 2025. PMID: 40002539 Free PMC article.
References
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

