Tetrodotoxin: The State-of-the-Art Progress in Characterization, Detection, Biosynthesis, and Transport Enrichment
- PMID: 39728106
- PMCID: PMC11676112
- DOI: 10.3390/md22120531
Tetrodotoxin: The State-of-the-Art Progress in Characterization, Detection, Biosynthesis, and Transport Enrichment
Abstract
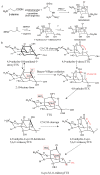
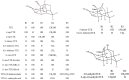
Tetrodotoxin (TTX) is a neurotoxin that binds to sodium channels and blocks sodium conduction. Importantly, TTX has been increasingly detected in edible aquatic organisms. Because of this and the lack of specific antidotes, TTX poisoning is now a major threat to public health. However, it is of note that ultra-low dose TTX is an excellent analgesic with great medicinal value. These contradictory effects highlight the need for further research to elucidate the impacts and functional mechanisms of TTX. This review summarizes the latest research progress in relation to TTX sources, analogs, mechanisms of action, detection methods, poisoning symptoms, therapeutic options, biosynthesis pathways, and mechanisms of transport and accumulation in pufferfish. This review also provides a theoretical basis for reducing the poisoning risks associated with TTX and for establishing an effective system for its use and management to ensure the safety of fisheries and human health.
Keywords: TTX biosynthesis; TTX transporter accumulation; puffer fish; tetrodotoxin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Searching for Paralytic Toxin, Tetrodotoxin, in Swedish Bivalve Shellfish.Mar Drugs. 2025 Jun 19;23(6):257. doi: 10.3390/md23060257. Mar Drugs. 2025. PMID: 40559666 Free PMC article.
-
Different role of TTX-sensitive voltage-gated sodium channel (NaV 1) subtypes in action potential initiation and conduction in vagal airway nociceptors.J Physiol. 2018 Apr 15;596(8):1419-1432. doi: 10.1113/JP275698. J Physiol. 2018. PMID: 29435993 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
Cited by
-
Searching for Paralytic Toxin, Tetrodotoxin, in Swedish Bivalve Shellfish.Mar Drugs. 2025 Jun 19;23(6):257. doi: 10.3390/md23060257. Mar Drugs. 2025. PMID: 40559666 Free PMC article.
-
Comparative Analysis of Intestinal Microbiota Between Tetrodotoxin-Containing and Tetrodotoxin-Free Takifugu rubripes.Mar Drugs. 2025 Mar 24;23(4):140. doi: 10.3390/md23040140. Mar Drugs. 2025. PMID: 40278261 Free PMC article.
References
-
- Suehiro M. Historical review on chemical and medical studies of globefish toxin before World War II. Yakushigaku Zasshi. 1994;29:428–434. - PubMed
-
- Kotipoyina H.R., Kong E.L., Chen R.J., Warrington S.J. Tetrodotoxin Toxicity. StatPearls Publishing LLC; Treasure Island, FL, USA: 2024. - PubMed
-
- Asakawa M., Matsumoto T., Umezaki K., Kaneko K., Yu X., Gomez-Delan G., Tomano S., Noguchi T., Ohtsuka S. Toxicity and toxin composition of the greater blue-ringed octopus Hapalochlaena lunulata from ishigaki island, okinawa prefecture, Japan. Toxins. 2019;11:245. doi: 10.3390/toxins11050245. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

