Butyrolactone-I from Marine Fungal Metabolites Mitigates Heat-Stress-Induced Apoptosis in IPEC-J2 Cells and Mice Through the ROS/PERK/CHOP Signaling Pathway
- PMID: 39728139
- PMCID: PMC11676246
- DOI: 10.3390/md22120564
Butyrolactone-I from Marine Fungal Metabolites Mitigates Heat-Stress-Induced Apoptosis in IPEC-J2 Cells and Mice Through the ROS/PERK/CHOP Signaling Pathway
Abstract
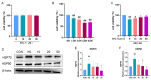
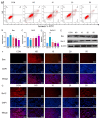
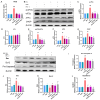
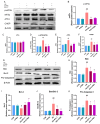
Heat stress poses a significant challenge to animal husbandry, contributing to oxidative stress, intestinal mucosal injury, and apoptosis, which severely impact animal health, growth, and production efficiency. The development of safe, sustainable, and naturally derived solutions to mitigate these effects is critical for advancing sustainable agricultural practices. Butyrolactone-I (BTL-I), a bioactive compound derived from deep-sea fungi (Aspergillus), shows promise as a functional feed additive to combat heat stress in animals. This study explored the protective effects of BTL-I against heat-stress-induced oxidative stress and apoptosis in IPEC-J2 cells and mice. Our findings demonstrated that BTL-I effectively inhibited the heat-stress-induced upregulation of HSP70 and HSP90, alleviating intestinal heat stress. Both in vitro and in vivo experiments revealed that heat stress increased intestinal cell apoptosis, with a significant upregulation of Bax/Bcl-2 expression, while BTL-I pretreatment significantly reduced apoptosis-related protein levels, showcasing its protective effects. Furthermore, BTL-I suppressed oxidative stress markers (ROS and MDA) while enhancing antioxidant activity (SOD levels). BTL-I also reduced the expression of p-PERK, p-eIF2α, ATF4, and CHOP, mitigating oxidative and endoplasmic reticulum stress in intestinal cells. In conclusion, BTL-I demonstrates the potential to improve animal resilience to heat stress, supporting sustainable livestock production systems. Its application as a natural, eco-friendly feed additive will contribute to the development of sustainable agricultural practices.
Keywords: Butyrolactone-I (BTL-I); HSP70 and HSP90 expression; endoplasmic reticulum stress; heat stress; intestinal apoptosis; oxidative stress.
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships construed as potential conflicts of interest.
Figures






Similar articles
-
Trehalose Attenuates Oxidative Stress and Endoplasmic Reticulum Stress-Mediated Apoptosis in IPEC-J2 Cells Subjected to Heat Stress.Animals (Basel). 2022 Aug 16;12(16):2093. doi: 10.3390/ani12162093. Animals (Basel). 2022. PMID: 36009683 Free PMC article.
-
Choline attenuates heat stress-induced oxidative injury and apoptosis in bovine mammary epithelial cells by modulating PERK/Nrf-2 signaling pathway.Mol Immunol. 2021 Jul;135:388-397. doi: 10.1016/j.molimm.2021.05.002. Epub 2021 May 19. Mol Immunol. 2021. PMID: 34022514
-
Crosstalk between Endoplasmic Reticulum Stress and Oxidative Stress in Heat Exposure-Induced Apoptosis Is Dependent on the ATF4-CHOP-CHAC1 Signal Pathway in IPEC-J2 Cells.J Agric Food Chem. 2021 Dec 29;69(51):15495-15511. doi: 10.1021/acs.jafc.1c03361. Epub 2021 Dec 17. J Agric Food Chem. 2021. PMID: 34919378
-
Hypoxia-inducible factor 1α inhibits heat stress-induced pig intestinal epithelial cell apoptosis through eif2α/ATF4/CHOP signaling.Sci Total Environ. 2024 May 10;924:171649. doi: 10.1016/j.scitotenv.2024.171649. Epub 2024 Mar 12. Sci Total Environ. 2024. PMID: 38485018
-
ROS-mediated PERK-eIF2α-ATF4 pathway plays an important role in arsenite-induced L-02 cells apoptosis via regulating CHOP-DR5 signaling.Environ Toxicol. 2020 Oct;35(10):1100-1113. doi: 10.1002/tox.22946. Epub 2020 Jun 7. Environ Toxicol. 2020. PMID: 32506763
References
-
- Zheng Y., Zhao Y., He W., Wang Y., Cao Z., Yang H., Wang W., Li S. Novel organic selenium source hydroxy-selenomethionine counteracts the blood-milk barrier disruption and inflammatory response of mice under heat stress. Front. Immunol. 2022;13:1054128. doi: 10.3389/fimmu.2022.1054128. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 32273077/National Natural Science Foundation of China
- 2019A1515011142/Natural Science Foundation of Guangdong Province, China
- 2022KCXTD014/Innovation Team Project of Guangdong Provincial Department of Education
- 040510052201/Guangdong Postgraduate Education Innovation Project
- JCYJ20220530162008018/National Natural Science Foundation of ShenZhen
LinkOut - more resources
Full Text Sources
Research Materials

