Decellularized Green and Brown Macroalgae as Cellulose Matrices for Tissue Engineering
- PMID: 39728190
- PMCID: PMC11677820
- DOI: 10.3390/jfb15120390
Decellularized Green and Brown Macroalgae as Cellulose Matrices for Tissue Engineering
Abstract
Scaffolds resembling the extracellular matrix (ECM) provide structural support for cells in the engineering of tissue constructs. Various material sources and fabrication techniques have been employed in scaffold production. Cellulose-based matrices are of interest due to their abundant supply, hydrophilicity, mechanical strength, and biological inertness. Terrestrial and marine plants offer diverse morphologies that can replicate the ECM of various tissues and be isolated through decellularization protocols. In this study, three marine macroalgae species-namely Durvillaea poha, Ulva lactuca, and Ecklonia radiata-were selected for their morphological variation. Low-intensity, chemical treatments were developed for each species to maintain native cellulose structures within the matrices while facilitating the clearance of DNA and pigment. Scaffolds generated from each seaweed species were non-toxic for human dermal fibroblasts but only the fibrous inner layer of those derived from E. radiata supported cell attachment and maturation over the seven days of culture. These findings demonstrate the potential of E. radiata-derived cellulose scaffolds for skin tissue engineering and highlight the influence of macroalgae ECM structures on decellularization efficiency, cellulose matrix properties, and scaffold utility.
Keywords: cellulose; decellularization; fibroblast; macroalgae; matrix; scaffold; seaweed; skin; tissue engineering.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


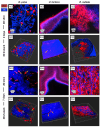
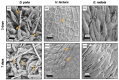
Similar articles
-
Seaweed cellulose scaffolds derived from green macroalgae for tissue engineering.Sci Rep. 2021 Jun 4;11(1):11843. doi: 10.1038/s41598-021-90903-2. Sci Rep. 2021. PMID: 34088909 Free PMC article.
-
Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices.Acta Biomater. 2016 Dec;46:91-100. doi: 10.1016/j.actbio.2016.09.043. Epub 2016 Sep 29. Acta Biomater. 2016. PMID: 27693690 Free PMC article.
-
Fabrication of a multi-layered decellularized amniotic membranes as tissue engineering constructs.Tissue Cell. 2022 Feb;74:101693. doi: 10.1016/j.tice.2021.101693. Epub 2021 Nov 19. Tissue Cell. 2022. PMID: 34856451
-
Decellularized Cell-Secreted Extracellular Matrices as Biomaterials for Tissue Engineering.Small Sci. 2024 Dec 6;5(2):2400335. doi: 10.1002/smsc.202400335. eCollection 2025 Feb. Small Sci. 2024. PMID: 40213064 Free PMC article. Review.
-
An overview of the production of tissue extracellular matrix and decellularization process.Cell Tissue Bank. 2024 Mar;25(1):369-387. doi: 10.1007/s10561-023-10112-1. Epub 2023 Oct 9. Cell Tissue Bank. 2024. PMID: 37812368 Review.
References
-
- Lanza R., Langer R., Vacanti J.P., Atala A., editors. Principles of Tissue Engineering. 5th ed. Academic Press; Cambridge, MA, USA: 2020. pp. 1–1635.
-
- O’Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today. 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. - DOI
-
- Rahmati M., Mills D.K., Urbanska A.M., Saeb M.R., Venugopal J.R., Ramakrishna S., Mozafari M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021;117:100721. doi: 10.1016/j.pmatsci.2020.100721. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

