Innovative Polymeric Biomaterials for Intraocular Lenses in Cataract Surgery
- PMID: 39728191
- PMCID: PMC11678174
- DOI: 10.3390/jfb15120391
Innovative Polymeric Biomaterials for Intraocular Lenses in Cataract Surgery
Abstract
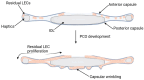
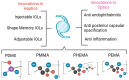
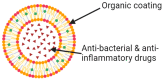
Intraocular lenses (IOLs) play a pivotal role in restoring vision following cataract surgery. The evolution of polymeric biomaterials has been central to addressing challenges such as biocompatibility, optical clarity, mechanical stability, and resistance to opacification. This review explores essential requirements for IOL biomaterials, emphasizing their ability to mitigate complications like posterior capsule opacification (PCO) and dysphotopsias while maintaining long-term durability and visual quality. Traditional polymeric materials, including polymethyl methacrylate (PMMA), silicone, and acrylic polymers, are critically analyzed alongside cutting-edge innovations such as hydrogels, shape memory polymers, and light-adjustable lenses (LALs). Advances in polymer engineering have enabled these materials to achieve enhanced flexibility, transparency, and biocompatibility, driving their adoption in modern IOL design. Functionalization strategies, including surface modifications and drug-eluting designs, highlight advancements in preventing inflammation, infection, and other complications. The incorporation of UV-blocking and blue-light-filtering agents is also examined for their potential in reducing retinal damage. Furthermore, emerging technologies like nanotechnology and smart polymer-based biomaterials offer promising avenues for personalized, biocompatible IOLs with enhanced performance. Clinical outcomes, including visual acuity, contrast sensitivity, and patient satisfaction, are evaluated to provide an understanding of the current advancements and limitations in IOL development. We also discuss the current challenges and future directions, underscoring the need for cost-effective, innovative polymer-based solutions to optimize surgical outcomes and improve patients' quality of life.
Keywords: antibacterial materials; biocompatibility; biosensors and biodetection; cataract surgery; hydrogels; intraocular lenses; nanomedicines; polymeric biomaterials; shape memory polymers; surface modifications.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Intraocular lenses for the treatment of age-related cataracts: an evidence-based analysis.Ont Health Technol Assess Ser. 2009;9(15):1-62. Epub 2009 Oct 1. Ont Health Technol Assess Ser. 2009. PMID: 23074519 Free PMC article.
-
Update on fixation of rigid and foldable posterior chamber intraocular lenses. Part II: Choosing the correct haptic fixation and intraocular lens design to help eradicate posterior capsule opacification.Ophthalmology. 1999 May;106(5):891-900. doi: 10.1016/S0161-6420(99)00506-0. Ophthalmology. 1999. PMID: 10328386
-
Efficacy of different intraocular lens materials and optic edge designs in preventing posterior capsular opacification: a meta-analysis.Am J Ophthalmol. 2007 Mar;143(3):428-36. doi: 10.1016/j.ajo.2006.11.045. Epub 2006 Dec 28. Am J Ophthalmol. 2007. PMID: 17224119
-
[Heparin Surface-Modified Poly(methylmethacrylate) and Foldable Hydrophobic Acrylic Intraocular Lenses in Cataract Patients with Acquired Immune Deficiency Syndrome and CMV-Retinitis].Klin Monbl Augenheilkd. 2004 Jan;221(1):40-6. doi: 10.1055/s-2003-812636. Klin Monbl Augenheilkd. 2004. PMID: 14745677 Clinical Trial. German.
-
Trifocal intraocular lenses versus bifocal intraocular lenses after cataract extraction among participants with presbyopia.Cochrane Database Syst Rev. 2020 Jun 18;6(6):CD012648. doi: 10.1002/14651858.CD012648.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Jan 27;1:CD012648. doi: 10.1002/14651858.CD012648.pub3. PMID: 32584432 Free PMC article. Updated.
References
Publication types
LinkOut - more resources
Full Text Sources

