Bioprosthetic Aortic Valve Degeneration After TAVR and SAVR: Incidence, Diagnosis, Predictors, and Management
- PMID: 39728274
- PMCID: PMC11676755
- DOI: 10.3390/jcdd11120384
Bioprosthetic Aortic Valve Degeneration After TAVR and SAVR: Incidence, Diagnosis, Predictors, and Management
Abstract
Bioprosthetic aortic valve degeneration (BAVD) is a significant clinical concern following both transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR). The increasing use of bioprosthetic valves in aortic valve replacement in younger patients and the subsequent rise in cases of BAVD are acknowledged in this review which aims to provide a comprehensive overview of the incidence, diagnosis, predictors, and management of BAVD. Based on a thorough review of the existing literature, this article provides an updated overview of the biological mechanisms underlying valve degeneration, including calcification, structural deterioration, and inflammatory processes and addresses the various risk factors contributing to BAVD, such as patient demographics, comorbidities, and procedural variables. The difficulties in early detection and accurate diagnosis of BAVD are discussed with an emphasis on the need for improved imaging techniques. The incidence and progression of BAVD in patients undergoing TAVR versus SAVR are compared, providing insights into the differences and similarities between the two procedures and procedural impacts on valve longevity. The current strategies for managing BAVD, including re-intervention options of redo surgery and valve-in-valve TAVR, along with emerging treatments are discussed. The controversies in the existing literature are highlighted to offer directions for future investigations to enhance the understanding and management of BAVD.
Keywords: aortic stenosis; aortic valve replacement; bioprosthetic aortic valve; structural valve degeneration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
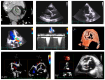
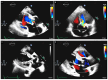
References
-
- Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Fleisher L.A., Jneid H., Mack M.J., McLeod C.J., O’Gara P.T., et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. doi: 10.1161/CIR.0000000000000503. - DOI - PubMed
-
- Cartlidge T.R.G., Doris M.K., Sellers S.L., Pawade T.A., White A.C., Pessotto R., Kwiecinski J., Fletcher A., Alcaide C., Lucatelli C., et al. Detection and Prediction of Bioprosthetic Aortic Valve Degeneration. J. Am. Coll. Cardiol. 2019;73:1107–1119. doi: 10.1016/j.jacc.2018.12.056. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

