Endothelial Dysfunction and Cardiovascular Disease: Hyperbaric Oxygen Therapy as an Emerging Therapeutic Modality?
- PMID: 39728298
- PMCID: PMC11677558
- DOI: 10.3390/jcdd11120408
Endothelial Dysfunction and Cardiovascular Disease: Hyperbaric Oxygen Therapy as an Emerging Therapeutic Modality?
Abstract
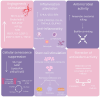
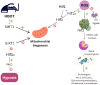
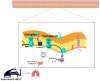
Maintaining the physiological function of the vascular endothelium and endothelial glycocalyx is crucial for the prevention of cardiovascular disease, which is one of the leading causes of morbidity and mortality worldwide. Damage to these structures can lead to atherosclerosis, hypertension, and other cardiovascular problems, especially in individuals with risk factors such as diabetes and obesity. Endothelial dysfunction is associated with ischemic disease and has a negative impact on overall cardiovascular health. The aim of this review was to comprehensively summarize the crucial role of the vascular endothelium and glycocalyx in cardiovascular health and associated thrombo-inflammatory conditions. It highlights how endothelial dysfunction, influenced by factors such as diabetes, chronic kidney disease, and obesity, leads to adverse cardiovascular outcomes, including heart failure. Recent evidence suggests that hyperbaric oxygen therapy (HBOT) may offer therapeutic benefits in the treatment of cardiovascular risk factors and disease. This review presents the current evidence on the mechanisms by which HBOT promotes angiogenesis, shows antimicrobial and immunomodulatory effects, enhances antioxidant defenses, and stimulates stem cell activity. The latest findings on important topics will be presented, including the effects of HBOT on endothelial dysfunction, cardiac function, atherosclerosis, plaque stability, and endothelial integrity. In addition, the role of HBOT in alleviating cardiovascular risk factors such as hypertension, aging, obesity, and glucose metabolism regulation is discussed, along with its impact on inflammation in cardiovascular disease and its potential benefit in ischemia-reperfusion injury. While HBOT demonstrates significant therapeutic potential, the review also addresses potential risks associated with excessive oxidative stress and oxygen toxicity. By combining information on the molecular mechanisms of HBOT and its effects on the maintenance of vascular homeostasis, this review provides valuable insights into the development of innovative therapeutic strategies aimed at protecting and restoring endothelial function to prevent and treat cardiovascular diseases.
Keywords: cardiovascular diseases; endothelial dysfunction; endothelial glycocalyx; endothelium; hyperbaric oxygen therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Yamaoka-Tojo M. Vascular Endothelial Glycocalyx as a Mechanism of Vascular Endothelial Dysfunction and Atherosclerosis. World. J. Cardiovasc. Dis. 2020;10:731–749. doi: 10.4236/wjcd.2020.1010070. - DOI
Publication types
LinkOut - more resources
Full Text Sources

