Evaluation of Fifteen 5,6-Dihydrotetrazolo[1,5- c]quinazolines Against Nakaseomyces glabrata: Integrating In Vitro Studies, Molecular Docking, QSAR, and In Silico Toxicity Assessments
- PMID: 39728312
- PMCID: PMC11728297
- DOI: 10.3390/jof10120816
Evaluation of Fifteen 5,6-Dihydrotetrazolo[1,5- c]quinazolines Against Nakaseomyces glabrata: Integrating In Vitro Studies, Molecular Docking, QSAR, and In Silico Toxicity Assessments
Abstract
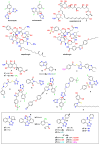
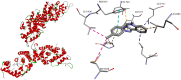
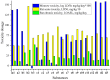
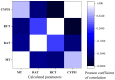
Nakaseomyces glabrata (Candida glabrata), the second most prevalent Candida pathogen globally, has emerged as a major clinical threat due to its ability to develop high-level azole resistance. In this study, two new 5,6-dihydrotetrazolo[1,5-c]quinazoline derivatives (c11 and c12) were synthesized and characterized using IR, LC-MS, 1H, and 13C NMR spectra. Along with 13 previously reported analogues, these compounds underwent in vitro antifungal testing against clinical N. glabrata isolates using a serial dilution method (0.125-64 mg/L). Remarkably, compounds c5 and c1 exhibited potent antifungal activity, with minimum inhibitory concentrations of 0.37 μM and 0.47 μM, respectively-about a 20-fold improvement in μM concentration over standard drugs like amphotericin B, caspofungin, and micafungin. A detailed structure-activity relationship analysis revealed crucial molecular features enhancing antifungal potency. Extensive molecular docking studies across 18 protein targets explored potential binding pockets and affinities of the lead compounds. A robust 3D-QSAR model, incorporating molecular descriptors Mor26m and Mor29e, displayed good predictive ability for antifungal activity. In silico predictions indicated an absence of herbicidal effect, negligible environmental toxicity (to honeybees, avian species, and aquatic organisms), and mild human toxicity concerns for these compounds. This comprehensive approach aims to develop novel and effective antifungal compounds against the clinically relevant pathogen N. glabrata.
Keywords: 5,6-dihydrotetrazolo[1,5-c]quinazolines; Nakaseomyces glabrata; QSAR; antifungal activity; molecular docking; toxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Antifungal susceptibility profile of invasive Candida glabrata isolates (2009-2020) from a tertiary care hospital laboratory in Pakistan.J Med Microbiol. 2021 Dec;70(12). doi: 10.1099/jmm.0.001459. J Med Microbiol. 2021. PMID: 34878377
-
5,6-Dihydrotetrazolo[1,5-c]quinazolines: Toxicity prediction, synthesis, antimicrobial activity, molecular docking, and perspectives.Arch Pharm (Weinheim). 2023 Jun;356(6):e2300029. doi: 10.1002/ardp.202300029. Epub 2023 Mar 2. Arch Pharm (Weinheim). 2023. PMID: 36864600
-
Molecular characterization and antifungal susceptibility testing of Candida nivariensis from blood samples - an Iranian multicentre study and a review of the literature.J Med Microbiol. 2019 May;68(5):770-777. doi: 10.1099/jmm.0.000963. Epub 2019 Mar 29. J Med Microbiol. 2019. PMID: 30924763 Review.
-
Metabolite profiling, antifungal, biofilm formation prevention and disruption of mature biofilm activities of Erythrina senegalensis stem bark extract against Candida albicans and Candida glabrata.PLoS One. 2022 Nov 28;17(11):e0278096. doi: 10.1371/journal.pone.0278096. eCollection 2022. PLoS One. 2022. PMID: 36441750 Free PMC article.
References
-
- Anderson H.W. Yeast-Like Fungi of the Human Intestinal Tract. J. Infect. Dis. 1917;21:341–386. doi: 10.1093/infdis/21.4.341. - DOI
-
- Pappas P.G., Kauffman C.A., Andes D.R., Clancy C.J., Marr K.A., Ostrosky-Zeichner L., Reboli A.C., Schuster M.G., Vazquez J.A., Walsh T.J., et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. - DOI - PMC - PubMed
-
- Ryan P., Motherway C., Powell J., Elsaka A., Sheikh A.A., Jahangir A., O’Connell N.H., Dunne C.P. Candidaemia in an Irish Intensive Care Unit Setting between 2004 and 2018 Reflects Increased Incidence of Candida glabrata. J. Hosp. Infect. 2019;102:347–350. doi: 10.1016/j.jhin.2019.01.017. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

