Evaluation of Lipid Changes During the Drying Process of Cordyceps sinensis by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)-Based Lipidomics Technique
- PMID: 39728352
- PMCID: PMC11677378
- DOI: 10.3390/jof10120855
Evaluation of Lipid Changes During the Drying Process of Cordyceps sinensis by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)-Based Lipidomics Technique
Abstract
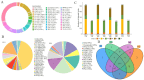
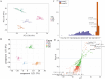
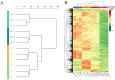
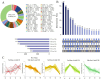
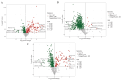
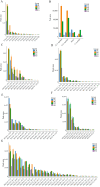
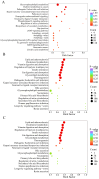
Comprehensive analysis of the lipid content in Cordyceps sinensis samples is essential for optimizing their effective use. Understanding the lipid profile can significantly enhance the application of this valuable fungus across various fields, including nutrition and medicine. However, to date, there is limited knowledge regarding the effects of different drying methods on the quality of lipids present in Cordyceps sinensis. In this study, we employed a broadly targeted lipidomic strategy to conduct a comprehensive analysis of the lipid composition in Cordyceps sinensis subjected to various drying methods. A comprehensive analysis identified a total of 765 distinct lipid species from fresh Cordyceps sinensis (FC), vacuum-freeze-dried Cordyceps sinensis (VG), oven-dried Cordyceps sinensis (OG), and air-dried Cordyceps sinensis (AG). Among these, glycerophospholipids (GP) were the most abundant, followed by glycerides (GL) and sphingolipids (SP). In this study, a total of 659 lipids demonstrated statistically significant differences, as indicated by a p-value (p) < 1. Among these lipids, triglycerides (TG) exhibited the highest concentration, followed by several others, including ceramide-ascorbic acid (Cer-AS), phosphatidylethanolamine (PE), lysophosphatidylcholine (LPC), and phosphatidylserine (PS). OG was the fastest drying method; however, PCA and OPLS-DA analyses indicated that the most significant changes in the lipids of Cordyceps sinensis were observed under the OG method. Specifically, 517 differentially accumulated lipids were significantly down-regulated, while only 10 lipids were significantly up-regulated. This disparity may be attributed to the degradation and oxidation of lipids. The metabolic pathways of glycerolipid, glycerophospholipid, and cholesterol are critical during the drying process of Cordyceps sinensis. This study provides valuable insights that can enhance quality control and offer guidelines for the appropriate storage of this medicinal fungus.
Keywords: Cordyceps sinensis; drying methods; lipidomic; multiple reaction monitoring (MRM); oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tang C., Li X., Wang T., Wang J., Xiao M., He M., Chang X., Fan Y., Li Y. Characterization of Metabolite Landscape Distinguishes Medicinal Fungus Cordyceps sinensis and other Cordyceps by UHPLC-Q Exactive HF-X Untargeted Metabolomics. Molecules. 2023;28:7745. doi: 10.3390/molecules28237745. - DOI - PMC - PubMed
Grants and funding
- (LHZX-2022-01)/the Chinese Academy of Sciences-People's Government of Qinghai Province on Sanjiangyuan National Park
- (QHCY-2023-057)/The Protective Harvesting and Utilization Project for Ophiocordyceps sinensis in Qinghai Province
- (2021-SF-A4)/the major science and technology projects of the Qinghai Province
- (2024-1-NCC-0058)/Qinghai Province Science and Technology Commissioner Special Project
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

