Biotransformation of the Fluoroquinolone Antibiotic, Levofloxacin, by the Free and Immobilized Secretome of Coriolopsis gallica
- PMID: 39728357
- PMCID: PMC11677856
- DOI: 10.3390/jof10120861
Biotransformation of the Fluoroquinolone Antibiotic, Levofloxacin, by the Free and Immobilized Secretome of Coriolopsis gallica
Abstract
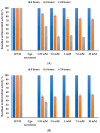
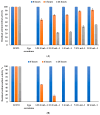
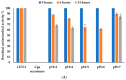
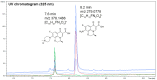
Antibiotics play a crucial role in human and animal medical healthcare, but widespread use and overuse of antibiotics poses alarming health and environmental issues. Fluoroquinolones constitute a class of antibiotics that has already become ubiquitous in the environment, and their increasing use and high persistence prompt growing concern. Here we investigated a fungal secretome prepared from the white-rot fungus Coriolopsis gallica, which is able to effectively degrade the environmentally persistent fluoroquinolone, levofloxacin. We tested various physical-chemical factors such as concentrations of 1-hydroxybenzotriazol (HBT), of enzyme, and of antibiotic, and pH and temperature of the reaction for biotransformation of the antibiotic. We compared the free with the immobilized Coriolopsis gallica secretome proteins, and analyzed the collective reaction products for residual activity against E. coli (growth inhibition test). We also performed HPLC analysis. The results show that treatment with the free secretome yielded a highest removal efficiency of 50 mg L-1 levofloxacin in the presence of 2.5 mM HBT, whereas the immobilized secretome was only able to degrade 10 mg L-1 levofloxacin with the same concentration of mediator, but presenting the advantage of being reusable.
Keywords: Coriolopsis gallica secretome; alginate immobilization; biotransformation; fluoroquinolone; fungal bioremediation; levofloxacin; mediator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lulijwa R., Rupia E.J., Alfaro A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020;12:640–663. doi: 10.1111/raq.12344. - DOI
LinkOut - more resources
Full Text Sources

