Effects of the Interaction of Salinity and Rare Earth Elements on the Health of Mytilus galloprovincialis: The Case of Praseodymium and Europium
- PMID: 39728416
- PMCID: PMC11676891
- DOI: 10.3390/jox14040108
Effects of the Interaction of Salinity and Rare Earth Elements on the Health of Mytilus galloprovincialis: The Case of Praseodymium and Europium
Abstract
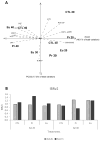
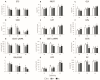
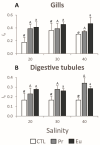
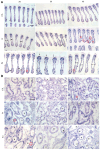
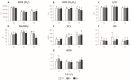
The growing use of products containing rare earth elements (REEs) may lead to higher environmental emissions of these elements, which can potentially enter aquatic systems. Praseodymium (Pr) and europium (Eu) are widely used REEs with various applications. However, their ecotoxicological impacts remain largely unexplored, with poorly understood risks to wildlife. Moreover, organisms also face environmental stressors like salinity fluctuations, and the nature of the interaction between salinity variations and contaminants is not yet clear. Therefore, this study aimed to evaluate the influence of salinity shifts on the impacts of Pr and Eu on adult mussels and the sperm of the species Mytilus galloprovincialis after 28 days and 30 min of exposure, respectively. To do so, biochemical and histopathological alterations were evaluated in adults, while biochemical and physiological changes were analysed in sperm. Additionally, the Integrated Biological Index (IBR) was calculated to understand the overall impact of each treatment. The results showed that adult mussels were most affected when exposed to the combination of high salinity and each element, which altered the behaviour of defence mechanisms causing redox imbalance and cellular damage. On the other hand, sperm demonstrated sensitivity to specific REE-salinity combinations, particularly Pr at lower salinity and Eu at higher salinity. These specific treatments elicited changes in sperm motility and velocity: Pr 20 led to a higher production of O2- and a decrease in velocity, while Eu 40 resulted in reduced motility and an increase in irregular movement. At both lower and higher salinity levels, exposure to Eu caused similar sensitivities in adults and sperm, reflected by comparable IBR scores. In contrast, Pr exposure induced greater alterations in sperm than in adult mussels at lower salinity, whereas the reverse was observed at higher salinity. These findings suggest that reproductive success and population dynamics could be modulated by interactions between salinity levels and REE pollution, highlighting the need for further investigation into how REEs and environmental factors interact. This study offers valuable insights to inform policymakers about the potential risks of REE contamination, emphasising the importance of implementing environmental regulations and developing strategies to mitigate the impact of these pollutants.
Keywords: adult mussels; biochemistry; histopathology; rare earth elements; salinity; sperm.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
From the cellular to tissue alterations induced by two rare earth elements in the mussel species Mytilus galloprovincialis: Comparison between exposure and recovery periods.Sci Total Environ. 2024 Mar 10;915:169754. doi: 10.1016/j.scitotenv.2023.169754. Epub 2023 Dec 30. Sci Total Environ. 2024. PMID: 38163599
-
Praseodymium and warming interactions in mussels: Comparison between observed and predicted results.Sci Total Environ. 2024 Jul 15;934:172893. doi: 10.1016/j.scitotenv.2024.172893. Epub 2024 Apr 29. Sci Total Environ. 2024. PMID: 38692321
-
Salinity modulation of neodymium and dysprosium toxicity in mussels: A comprehensive analysis of adult and sperm responses.Sci Total Environ. 2025 Jan 10;959:177995. doi: 10.1016/j.scitotenv.2024.177995. Epub 2024 Dec 24. Sci Total Environ. 2025. PMID: 39721537
-
Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants.Sci Total Environ. 2018 Sep 15;636:299-313. doi: 10.1016/j.scitotenv.2018.04.235. Epub 2018 Apr 27. Sci Total Environ. 2018. PMID: 29709849 Review.
-
Molecular insights into rare earth element (REE)-mediated phytotoxicity and its impact on human health.Environ Sci Pollut Res Int. 2023 Aug;30(36):84829-84849. doi: 10.1007/s11356-023-27299-1. Epub 2023 May 3. Environ Sci Pollut Res Int. 2023. PMID: 37138125 Review.
References
-
- Arienzo M., Ferrara L., Trifuoggi M., Toscanesi M. Advances in the Fate of Rare Earth Elements, REE, in Transitional Environments: Coasts and Estuaries. Water. 2022;14:401. doi: 10.3390/w14030401. - DOI
-
- Sneller F.E.C., Kalf D.F., Weltje L., Van Wezel A.P. Maximum Permissible Concentrations and Negligible Concentrations for Rare Earth Elements (REEs) Volume 601. RIVM; Bilthoven, The Netherlands: 2000. 66p RIVM Repport 601501011.
-
- Balaram V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019;10:1285–1303. doi: 10.1016/j.gsf.2018.12.005. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

