Effects of Ammonia Stress on Liver Tissue Structure, Enzyme Activities, and Metabolome of Juvenile Largemouth Bass Micropterus salmoides
- PMID: 39728430
- PMCID: PMC11678563
- DOI: 10.3390/metabo14120649
Effects of Ammonia Stress on Liver Tissue Structure, Enzyme Activities, and Metabolome of Juvenile Largemouth Bass Micropterus salmoides
Abstract
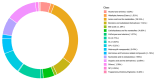
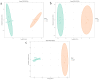
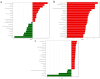
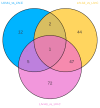
Background: Ammonia, a ubiquitous contaminant in aquatic ecosystems, poses multifaceted threats to fish species at elevated concentrations. Methods: In order to investigate the toxic effects of chronic ammonia stress on the liver of juvenile Micropterus salmoides, the present experiment was conducted to investigate the differences in changes in liver tissue structure, enzyme activities, and metabolomes after 28 days of ammonia exposure (0, 4, 8, and 16 mg/L). Results: The findings revealed that ammonia exposure induced significant oxidative stress in the liver, manifesting in decreased activities of antioxidant enzymes SOD and GSH-Px, elevated levels of GSH, GST, and MDA, and heightened activities of immune enzymes LZM, ALP, and ACP. An increase in ammonia concentration exacerbated liver tissue damage. Metabolome analysis further unveiled perturbations in liver metabolites of Micropterus salmoides exposed to ammonia, with Ala-His emerging as a potentially pivotal functional substance under chronic stress. Specifically, the 4 mg/L group responded to ammonia toxicity by augmenting GSH and L-Carnosine levels, the 8 mg/L group detoxified via upregulation of L-Glutamine, and the 16 mg/L group mitigated toxicity through the urea synthesis pathway. Conclusions: This research offers preliminary insights into the toxicological responses of Micropterus salmoides under chronic ammonia stress. It is suggested that the duration of ammonia concentration exceeding 4 mg/L in high-density aquaculture should not exceed 7 days.
Keywords: ammonia toxicity; immune enzymes; metabolites; oxidative stress; tissue damage.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

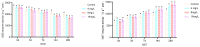
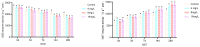




References
-
- Zhang W., Xia S., Zhu J., Miao L., Ren M., Lin Y., Ge X., Sun S. Growth performance, physiological response and histology changes of juvenile blunt snout bream, Megalobrama amblycephala exposed to chronic ammonia. Aquaculture. 2019;506:424–436. doi: 10.1016/j.aquaculture.2019.03.072. - DOI
-
- Sun Z., Wang S., Zhang M., Jiang H., Li M. Chronic toxicity study of ammonia exposure in juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture. 2023;567:739266. doi: 10.1016/j.aquaculture.2023.739266. - DOI
Grants and funding
- No. cqaas2023sjczqn036/the Chongqing Academy of Agricultural Sciences Municipal Finance Youth Special Fund Project
- No. 2022YFD2001700/the Key Research and Development Plan of the Ministry of Science and Technology
- No. CQFTIU2024-10/the Research on Engineered High-efficiency Recirculating Water Aquaculture Model
- No. CQMAITS202315/the Chongqing Modern Agricultural Industry Technology System
LinkOut - more resources
Full Text Sources
Research Materials

