Longitudinal Metabolomics Reveals Metabolic Dysregulation Dynamics in Patients with Severe COVID-19
- PMID: 39728437
- PMCID: PMC11676849
- DOI: 10.3390/metabo14120656
Longitudinal Metabolomics Reveals Metabolic Dysregulation Dynamics in Patients with Severe COVID-19
Abstract
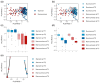
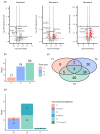
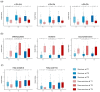
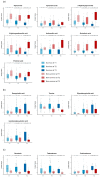
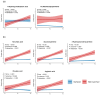
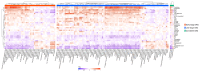
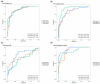
Background/Objective: A dysregulated metabolism has been studied as a key aspect of the COVID-19 pathophysiology, but its longitudinal progression in severe cases remains unclear. In this study, we aimed to investigate metabolic dysregulation over time in patients with severe COVID-19 requiring mechanical ventilation (MV). Methods: In this single-center, prospective, observational study, we obtained 236 serum samples from 118 adult patients on MV in an ICU. The metabolite measurements were performed using capillary electrophoresis Fourier transform mass spectrometry, and we categorized the sampling time points into three time zones to align them with the disease progression: time zone 1 (T1) (the hyperacute phase, days 1-3 post-MV initiation), T2 (the acute phase, days 4-14), and T3 (the chronic phase, days 15-30). Using volcano plots and enrichment pathway analyses, we identified the differential metabolites (DMs) and enriched pathways (EPs) between the survivors and non-survivors for each time zone. The DMs and EPs were further grouped into early-stage, late-stage, and consistent groups based on the time zones in which they were detected. Results: With the 566 annotated metabolites, we identified 38 DMs and 17 EPs as the early-stage group, which indicated enhanced energy production in glucose, amino acid, and fatty acid metabolisms in non-survivors. As the late-stage group, 84 DMs and 10 EPs showed upregulated sphingolipid, taurine, and tryptophan-kynurenine metabolisms with downregulated steroid hormone synthesis in non-survivors. Three DMs and 23 EPs in the consistent group showed more pronounced dysregulation in the dopamine and arachidonic acid metabolisms across all three time zones in non-survivors. Conclusions: This study elucidated the temporal differences in metabolic dysregulation between survivors and non-survivors of severe COVID-19, offering insights into its longitudinal progression and disease mechanisms.
Keywords: longitudinal metabolic dysregulation; severe COVID-19.
Conflict of interest statement
R.U. receives research funding from Human Metabolome Technologies, Inc. The funders played roles in the data analyses and interpretation.
Figures




References
-
- Termorshuizen F., Dongelmans D.A., Brinkman S., Bakhshi-Raiez F., Arbous M.S., de Lange D.W., van Bussel B.C.T., de Keizer N.F. Dutch COVID-19 Research Consortium Characteristics and Outcome of COVID-19 Patients Admitted to the ICU: A Nationwide Cohort Study on the Comparison between the Consecutive Stages of the COVID-19 Pandemic in the Netherlands, an Update. Ann. Intensive Care. 2024;14:11. doi: 10.1186/s13613-023-01238-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

