Structural and Functional Integration of Tissue-Nonspecific Alkaline Phosphatase Within the Alkaline Phosphatase Superfamily: Evolutionary Insights and Functional Implications
- PMID: 39728440
- PMCID: PMC11677397
- DOI: 10.3390/metabo14120659
Structural and Functional Integration of Tissue-Nonspecific Alkaline Phosphatase Within the Alkaline Phosphatase Superfamily: Evolutionary Insights and Functional Implications
Abstract
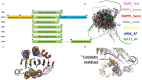
Phosphatases are enzymes that catalyze the hydrolysis of phosphate esters. They play critical roles in diverse biological processes such as extracellular nucleotide homeostasis, transport of molecules across membranes, intracellular signaling pathways, or vertebrate mineralization. Among them, tissue-nonspecific alkaline phosphatase (TNAP) is today increasingly studied, due to its ubiquitous expression and its ability to dephosphorylate a very broad range of substrates and participate in several different biological functions. For instance, TNAP hydrolyzes inorganic pyrophosphate (PPi) to allow skeletal and dental mineralization. Additionally, TNAP hydrolyzes pyridoxal phosphate to allow cellular pyridoxal uptake, and stimulate vitamin B6-dependent reactions. Furthermore, TNAP has been identified as a key enzyme in non-shivering adaptive thermogenesis, by dephosphorylating phosphocreatine in the mitochondrial creatine futile cycle. This latter recent discovery and others suggest that the list of substrates and functions of TNAP may be much longer than previously thought. In the present review, we sought to examine TNAP within the alkaline phosphatase (AP) superfamily, comparing its sequence, structure, and evolutionary trajectory. The AP superfamily, characterized by a conserved central folding motif of a mixed beta-sheet flanked by alpha-helices, includes six subfamilies: AP, arylsulfatases (ARS), ectonucleotide pyrophosphatases/phosphodiesterases (ENPP), phosphoglycerate mutases (PGM), phosphonoacetate hydrolases, and phosphopentomutases. Interestingly, TNAP and several ENPP family members appear to participate in the same metabolic pathways and functions. For instance, extra-skeletal mineralization in vertebrates is inhibited by ENPP1-mediated ATP hydrolysis into the mineralization inhibitor PPi, which is hydrolyzed by TNAP expressed in the skeleton. Better understanding how TNAP and other AP family members differ structurally will be very useful to clarify their complementary functions. Structurally, TNAP shares the conserved catalytic core with other AP superfamily members but has unique features affecting substrate specificity and activity. The review also aims to highlight the importance of oligomerization in enzyme stability and function, and the role of conserved metal ion coordination, particularly magnesium, in APs. By exploring the structural and functional diversity within the AP superfamily, and discussing to which extent its members exert redundant, complementary, or specific functions, this review illuminates the evolutionary pressures shaping these enzymes and their broad physiological roles, offering insights into TNAP's multifunctionality and its implications for health and disease.
Keywords: alkaline phosphatase (AP) superfamily; arylsulfatases (ARS); ectonucleotide pyrophosphatases/phosphodiesterases (ENPP); tissue-nonspecific alkaline phosphatase (TNAP).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Whyte M.P., Fujita K.P., Moseley S., Thompson D.D., McAlister W.H. Validation of a Novel Scoring System for Changes in Skeletal Manifestations of Hypophosphatasia in Newborns, Infants, and Children: The Radiographic Global Impression of Change Scale. J. Bone Miner. Res. 2018;33:868–874. doi: 10.1002/jbmr.3377. - DOI - PubMed
-
- Fedde K.N., Blair L., Silverstein J., Coburn S.P., Ryan L.M., Weinstein R.S., Waymire K., Narisawa S., Millán J.L., Macgregor G.R., et al. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J. Bone Miner. Res. 1999;14:2015–2026. doi: 10.1359/jbmr.1999.14.12.2015. - DOI - PMC - PubMed
-
- Schippers M., Post E., Eichhorn I., Langeland J., Beljaars L., Malo M.S., Hodin R.A., Millán J.L., Popov Y., Schuppan D., et al. Phosphate Groups in the Lipid A Moiety Determine the Effects of LPS on Hepatic Stellate Cells: A Role for LPS-Dephosphorylating Activity in Liver Fibrosis. Cells. 2020;9:2708. doi: 10.3390/cells9122708. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

