Circadian Influences on Brain Lipid Metabolism and Neurodegenerative Diseases
- PMID: 39728504
- PMCID: PMC11677446
- DOI: 10.3390/metabo14120723
Circadian Influences on Brain Lipid Metabolism and Neurodegenerative Diseases
Abstract
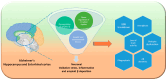
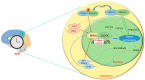
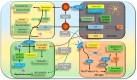
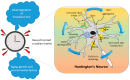
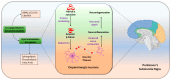
Circadian rhythms are intrinsic, 24 h cycles that regulate key physiological, mental, and behavioral processes, including sleep-wake cycles, hormone secretion, and metabolism. These rhythms are controlled by the brain's suprachiasmatic nucleus, which synchronizes with environmental signals, such as light and temperature, and consequently maintains alignment with the day-night cycle. Molecular feedback loops, driven by core circadian "clock genes", such as Clock, Bmal1, Per, and Cry, are essential for rhythmic gene expression; disruptions in these feedback loops are associated with various health issues. Dysregulated lipid metabolism in the brain has been implicated in the pathogenesis of neurological disorders by contributing to oxidative stress, neuroinflammation, and synaptic dysfunction, as observed in conditions such as Alzheimer's and Parkinson's diseases. Disruptions in circadian gene expression have been shown to perturb lipid regulatory mechanisms in the brain, thereby triggering neuroinflammatory responses and oxidative damage. This review synthesizes current insights into the interconnections between circadian rhythms and lipid metabolism, with a focus on their roles in neurological health and disease. It further examines how the desynchronization of circadian genes affects lipid metabolism and explores the potential mechanisms through which disrupted circadian signaling might contribute to the pathophysiology of neurodegenerative disorders.
Keywords: Alzheimer’s disease; brain function; cholesterol; circadian rhythm; fatty acid; lipid metabolism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Fagiani F., Di Marino D., Romagnoli A., Travelli C., Voltan D., Di Cesare Mannelli L., Racchi M., Govoni S., Lanni C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 2022;7:41. doi: 10.1038/s41392-022-00899-y. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

