Carbapenem-Resistant E. coli Adherence to Magnetic Nanoparticles
- PMID: 39728546
- PMCID: PMC11678732
- DOI: 10.3390/nano14242010
Carbapenem-Resistant E. coli Adherence to Magnetic Nanoparticles
Abstract
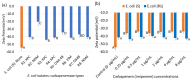
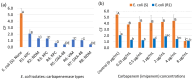
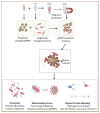
Carbapenem-resistant Enterobacterales (CRE) is an emerging global concern. Specifically, carbapenemase-producing (CP) E. coli strains in CRE have recently been found in clinical, environmental, and food samples worldwide, causing many hospitalizations and deaths. Their rapid identification and characterization are paramount in control, management options, and treatment choices. Thus, this study aimed to characterize the cell surface properties of carbapenem-resistant (R) E. coli isolates and their interaction with glycan-coated magnetic nanoparticles (gMNPs) compared with carbapenem-susceptible (S) E coli. This study used two groups of bacteria: The first group included E. coli (R) isolates harboring carbapenemases and had no antibiotic exposure. Their initial gMNP-cell binding capacity, with cell surface characteristics, was assessed. In the second group, one of the E. coli (R) isolates and E. coli (S) had long-term serial antibiotic exposure, which we used to observe their cell surface characteristics and gMNP interactions. Initially, cell surface characteristics (cell morphology and cell surface charge) of the E. coli isolates were evaluated using confocal laser scanning microscope (LSCM) and a Zetasizer, respectively. The interaction of gMNPs with the E. coli isolates was assessed through LSCM and transmission electron microscope (TEM). Further, the gMNP-cell attachment was quantified as a concentration factor (CF) through the standard plating method. The results showed that the CF values of all E. coli (R) were significantly different from those of E. coli (S), which could be due to the differences in cell characteristics. The E. coli (R) isolates displayed heterogeneous cell shapes (rod and round cells) and lower negative zeta potential (cell surface charge) values compared to E. coli (S). Further, this research identified the differences in the cell surface characteristics of E. coli (S) under carbapenem exposure, compared to unexposed E. coli (S) that impact their attachment capacity. The gMNPs captured more E. coli (S) cells compared to carbapenem-exposed E. coli (S) and all E. coli (R) isolates. This study clearly found that differences in cell surface characteristics impact their interaction with magnetic nanoparticles. The gained insights aid in further understanding adhesion mechanisms to develop or improve bacterial isolation techniques and diagnostic and treatment methods for CRE.
Keywords: CRE; MNP–cell interaction; cell morphology; gMNP; rapid isolation; surface charge.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Wellington E.M.H., Boxall A.B.A., Cross P., Feil E.J., Gaze W.H., Hawkey P.M., Johnson-Rollings A.S., Jones D.L., Lee N.M., Otten W., et al. The Role of the Natural Environment in the Emergence of Antibiotic Resistance in Gram-Negative Bacteria. Lancet Infect. Dis. 2013;13:155–165. doi: 10.1016/S1473-3099(12)70317-1. - DOI - PubMed
-
- Serwecińska L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water. 2020;12:3313. doi: 10.3390/w12123313. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

