Bacterial Outer Membrane Vesicle (OMV)-Encapsulated TiO2 Nanoparticles: A Dual-Action Strategy for Enhanced Radiotherapy and Immunomodulation in Oral Cancer Treatment
- PMID: 39728581
- PMCID: PMC11678132
- DOI: 10.3390/nano14242045
Bacterial Outer Membrane Vesicle (OMV)-Encapsulated TiO2 Nanoparticles: A Dual-Action Strategy for Enhanced Radiotherapy and Immunomodulation in Oral Cancer Treatment
Abstract
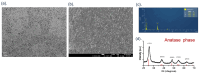
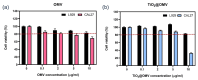
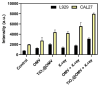
Oral squamous-cell carcinoma (OSCC) poses significant treatment challenges due to its high recurrence rates and the limitations of current therapies. Titanium dioxide (TiO2) nanoparticles are promising radiosensitizers, while bacterial outer membrane vesicles (OMVs) are known for their immunomodulatory properties. This study investigates the potential of OMV-encapsulated TiO2 nanoparticles (TiO2@OMV) to combine these effects for improved OSCC treatment. TiO2 nanoparticles were synthesized using a hydrothermal method and encapsulated within OMVs derived from Escherichia coli. The TiO2@OMV carriers were evaluated for their ability to enhance radiosensitivity and stimulate immune responses in OSCC cell lines. Reactive oxygen species (ROS) production, macrophage recruitment, and selective cytotoxicity toward cancer cells were assessed. TiO2@OMV demonstrated significant radiosensitization and immune activation compared to unencapsulated TiO2 nanoparticles. The system selectively induced cytotoxicity in OSCC cells, sparing normal cells, and enhanced ROS generation and macrophage-mediated antitumor responses. This study highlights TiO2@OMV as a dual-action therapeutic platform that synergizes radiotherapy and immunomodulation, offering a targeted and effective strategy for OSCC treatment. The approach could improve therapeutic outcomes and reduce the adverse effects associated with conventional therapies.
Keywords: TiO2 nanoparticles; oral cancer; outer membrane vesicles; radiotherapy.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures







References
-
- Pfister D.G., Spencer S., Adelstein D., Adkins D., Anzai Y., Brizel D.M., Bruce J.Y., Busse P.M., Caudell J.J., Cmelak A.J., et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020;18:873–898. doi: 10.6004/jnccn.2020.0031. - DOI - PubMed
Grants and funding
- FEMH-2024-C-013/Far Eastern Memorial Hospital
- NSTC 113-2314-B-418-010-M13/National Science and Technology Council
- NSTC 111-2221-E-A49-051-MY2/National Science and Technology Council
- NSTC 111-2811-E-A49A-007-MY2/National Science and Technology Council
- NSTC 113-2314-B-A49-065-MY3/National Science and Technology Council
LinkOut - more resources
Full Text Sources

