Fouling and Chemical Cleaning Strategies for Submerged Ultrafiltration Membrane: Synchronized Bench-Scale, Full-Scale, and Engineering Tests
- PMID: 39728701
- PMCID: PMC11679730
- DOI: 10.3390/membranes14120251
Fouling and Chemical Cleaning Strategies for Submerged Ultrafiltration Membrane: Synchronized Bench-Scale, Full-Scale, and Engineering Tests
Abstract
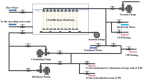
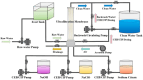
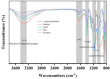
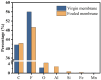
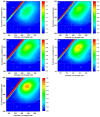
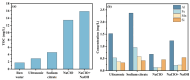
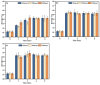
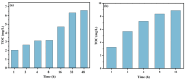
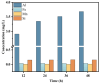
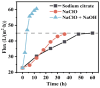
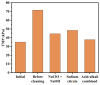
This study investigated membrane fouling issues associated with the operation of a submerged ultrafiltration membrane in a drinking water treatment plant (DWTP) and optimized the associated chemical cleaning strategies. By analyzing the surface components of the membrane foulant and the compositions of the membrane cleaning solution, the primary causes of membrane fouling were identified. Membrane fouling control strategies suitable for the DWTP were evaluated through chemical cleaning tests conducted for bench-scale, full-scale, and engineering cases. The results show that the membrane foulants were primarily composed of a mixture of inorganics and organics; the inorganics were mainly composed of Al and Si, while the organics were primarily humic acid (HA). Sodium citrate proved to be the most effective cleaning agent for inorganic fouling, which was mainly composed of Al, whereas sodium hypochlorite (NaClO) combined with sodium hydroxide (NaOH) showed the best removal efficiency for organic fouling, which predominantly consisted of HA and Si. However, sodium hypochlorite (NaClO) combined with sodium hydroxide (NaOH) showed the best removal efficiency for organic fouling and Si; organic fouling predominantly consisted of HA. Based on the bench-scale test results, flux recovery was verified in the full-scale system. Under a constant pressure of 30 kPa, the combined acid-alkali cleaning achieved the best flux recovery, restoring the flux from 22.8 L/(m2·h) to 66.75 L/(m2·h). In the engineering tests, combined acid-alkali cleaning yielded results consistent with those of the full-scale tests. In the practical engineering cleaning process, adopting a cleaning strategy of alkaline (NaClO + NaOH) cleaning followed by acidic (sodium citrate) cleaning can effectively solve the membrane fouling problem.
Keywords: chemical cleaning; flux recovery; fouling control strategies; membrane fouling; submerged ultrafiltration membrane.
Conflict of interest statement
Wenqing Yu is an employee of Zhejiang Supcon Information Co., Ltd. The other authors declare no conflicts of interest.
Figures


References
-
- Malkoske T.A., Bérubé P.R., Andrews R.C. Coagulation/flocculation prior to low pressure membranes in drinking water treatment: A review. Environ. Sci. Water Res. Technol. 2020;6:2993–3023. doi: 10.1039/D0EW00461H. - DOI
-
- Kumar R., Ismail A.F. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015;132:42042. doi: 10.1002/app.42042. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

