Effect of Monosaccharides Including Rare Sugars on the Bilayer Phase Behavior of Dimyristoylphosphatidylcholine
- PMID: 39728708
- PMCID: PMC11676506
- DOI: 10.3390/membranes14120258
Effect of Monosaccharides Including Rare Sugars on the Bilayer Phase Behavior of Dimyristoylphosphatidylcholine
Abstract
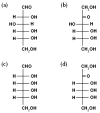
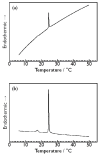
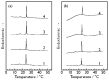
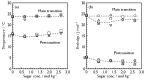
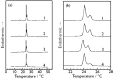
We observed bilayer phase transitions of dimyristoylphosphatidylcholine (DMPC) in aqueous solutions of four kinds of monosaccharides, namely, D-glucose, D-fructose, D-allose and D-psicose, using differential scanning calorimetry (DSC). D-allose (C3-epimer of D-glucose) and D-psicose (C3-epimer of D-fructose) are rare sugars. We performed DSC measurements using two types of sugar-containing sample dispersions of the DMPC vesicles: one is a normal sample dispersion with no concentration asymmetry between the inside and outside of the vesicles and the other is an unusual sample dispersion with a concentration asymmetry. DSC measurements using normal sample dispersions with different sugar concentrations revealed that the temperatures and transition enthalpies of the pre- and main transition of the DMPC bilayer membrane did not significantly depend on the sugar concentration for all monosaccharides. DSC measurements using the unusual sample dispersions demonstrated that the concentration asymmetry caused the splitting of the endothermic peak of the main transition similarly irrespective of the sort of monosaccharides present. From all these DSC results, we conclude that (i) most monosaccharide molecules exist in the bulk water phase, (ii) no specific interaction depending on the molecular structure of each monosaccharide directly occurs between the DMPC and each monosaccharide molecule, and (iii) almost all the effects of the monosaccharides observed in this study are understandable as the general colligative properties of solutions.
Keywords: bilayer membrane; differential scanning calorimetry; phase transition; phospholipid; rare sugar.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Effect of staphylococcal delta-lysin on the thermotropic phase behavior and vesicle morphology of dimyristoylphosphatidylcholine lipid bilayer model membranes. Differential scanning calorimetric, 31P nuclear magnetic resonance and Fourier transform infrared spectroscopic, and X-ray diffraction studies.Biochemistry. 1999 Dec 14;38(50):16514-28. doi: 10.1021/bi9913101. Biochemistry. 1999. PMID: 10600113
-
Interaction of the Saponin Aescin with Ibuprofen in DMPC Model Membranes.Mol Pharm. 2018 Oct 1;15(10):4446-4461. doi: 10.1021/acs.molpharmaceut.8b00421. Epub 2018 Aug 30. Mol Pharm. 2018. PMID: 30102549
-
Chain-melting phase transition and short-range molecular interactions in phospholipid foam bilayers.Adv Colloid Interface Sci. 2002 Feb 25;96(1-3):75-100. doi: 10.1016/s0001-8686(01)00076-8. Adv Colloid Interface Sci. 2002. PMID: 11908797
-
Effect of α-Tocopherol on the Microscopic Dynamics of Dimyristoylphosphatidylcholine Membrane.J Phys Chem B. 2016 Jan 14;120(1):154-63. doi: 10.1021/acs.jpcb.5b10417. Epub 2015 Dec 31. J Phys Chem B. 2016. PMID: 26673405
-
Solubilization of DMPC and DPPC vesicles by detergents below their critical micellization concentration: high-sensitivity differential scanning calorimetry, Fourier transform infrared spectroscopy and freeze-fracture electron microscopy reveal two interaction sites of detergents in vesicles.Biochim Biophys Acta. 1989 Sep 4;984(2):214-24. doi: 10.1016/0005-2736(89)90219-8. Biochim Biophys Acta. 1989. PMID: 2765550
References
-
- Ruocco M.J., Shipley G.G. Characterization of the sub-transition of hydrated dipalmitoylphosphatidylcholine bilayers: X-ray diffraction study. Biochim. Biophys. Acta. 1982;684:59–66. doi: 10.1016/0005-2736(82)90049-9. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

