Effects of Nicotine on the Thermodynamics and Phase Coexistence of Pulmonary Surfactant Model Membranes
- PMID: 39728717
- PMCID: PMC11678188
- DOI: 10.3390/membranes14120267
Effects of Nicotine on the Thermodynamics and Phase Coexistence of Pulmonary Surfactant Model Membranes
Abstract
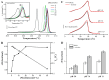
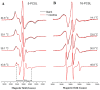
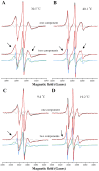
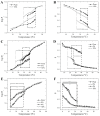
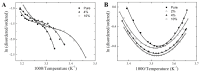
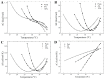
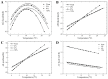
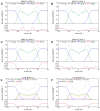
Phase separation is essential for membrane function, and alterations in phase coexistence by membrane-interacting molecules, such as nicotine, can impair membrane stability. With the increasing use of e-cigarettes, concerns have arisen about the impact of nicotine on pulmonary surfactants. Here, we used differential scanning calorimetry (DSC), molecular dynamics (MD) simulations, and electron spin resonance (ESR) to examine nicotine's effect on the phase coexistence of two surfactant models: pure DPPC and a DPPC/POPC/POPG mixture. Our DSC analysis revealed that nicotine interacts with both membranes, increasing enthalpy and entropy change during the phase transition. ESR revealed that nicotine affects membrane fluidity and packing of DPPC more effectively than the ternary mixture, especially near the surface. MD simulations showed that neutral nicotine resides in the mid-plane, while protonated nicotine remains near the surface. Nicotine binding to the membranes is dynamic, switching between bound and unbound states. Analysis via ESR/van't Hoff method revealed changes in the thermodynamics of phase coexistence, yielding distinct non-linear behavior. Nicotine altered the temperature dependence of the free energy, modifying the thermodynamic driving forces and the balance of non-covalent lipid interactions. These findings provide new insights into how nicotine influences pulmonary surfactant model membranes, with potential implications for surfactant function.
Keywords: DSC; ESR; MD simulations; drug-membrane interactions; nicotine; phase coexistence; pulmonary surfactant; van’t Hoff.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Non-linear van't Hoff behavior in pulmonary surfactant model membranes.Biochim Biophys Acta Biomembr. 2017 Jun;1859(6):1133-1143. doi: 10.1016/j.bbamem.2017.03.011. Epub 2017 Mar 21. Biochim Biophys Acta Biomembr. 2017. PMID: 28336314
-
A DSC and FTIR spectroscopic study of the effects of the epimeric 4-cholesten-3-ols and 4-cholesten-3-one on the thermotropic phase behaviour and organization of dipalmitoylphosphatidylcholine bilayer membranes: comparison with their 5-cholesten analogues.Chem Phys Lipids. 2014 Jan;177:71-90. doi: 10.1016/j.chemphyslip.2013.11.008. Epub 2013 Dec 1. Chem Phys Lipids. 2014. PMID: 24296232
-
Effect of phase ratio on van't Hoff analysis in reversed-phase liquid chromatography, and phase-ratio-independent estimation of transfer enthalpy.J Chromatogr A. 2003 Jun 27;1003(1-2):101-11. doi: 10.1016/s0021-9673(03)00846-x. J Chromatogr A. 2003. PMID: 12899299
-
Thermodynamics of surfactants, block copolymers and their mixtures in water: the role of the isothermal calorimetry.Int J Mol Sci. 2009 Jun 29;10(7):2873-2895. doi: 10.3390/ijms10072873. Int J Mol Sci. 2009. PMID: 19742173 Free PMC article. Review.
-
Role of lipid ordered/disordered phase coexistence in pulmonary surfactant function.Biochim Biophys Acta. 2012 Nov;1818(11):2550-62. doi: 10.1016/j.bbamem.2012.05.024. Epub 2012 May 31. Biochim Biophys Acta. 2012. PMID: 22659676 Review.
Cited by
-
DPPC Membrane Under Lateral Compression and Stretching to Extreme Limits: Phase Transitions and Rupture.Membranes (Basel). 2025 May 26;15(6):161. doi: 10.3390/membranes15060161. Membranes (Basel). 2025. PMID: 40559340 Free PMC article.
References
-
- Lewis R.N.A.H., Mcelhaney R.N. The Mesomorphic Phase Behavior of Lipid Bilayers. In: Yeagle P.L., editor. The Structure of Biological Membranes. CRC Press; Boca Raton, FL, USA: 2012. pp. 177–201. The structure of biological membranes.
Grants and funding
- 2015/18390-5/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2015/50366-7/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2023/04532-9/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2014/00206-0/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 210.778/2021/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
LinkOut - more resources
Full Text Sources
Miscellaneous

