Stable Convergent Polyneuronal Innervation and Altered Synapse Elimination in Orbicularis oculi Muscles from Patients with Blepharospasm Responding Poorly to Recurrent Botulinum Type-A Neurotoxin Injections
- PMID: 39728764
- PMCID: PMC11728458
- DOI: 10.3390/toxins16120506
Stable Convergent Polyneuronal Innervation and Altered Synapse Elimination in Orbicularis oculi Muscles from Patients with Blepharospasm Responding Poorly to Recurrent Botulinum Type-A Neurotoxin Injections
Abstract
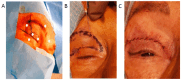
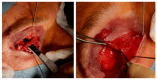
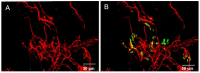
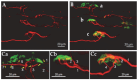
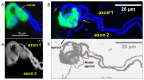
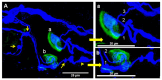
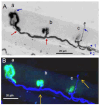
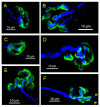
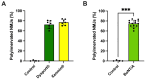
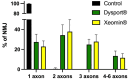
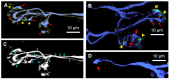
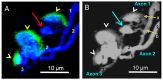
Botulinum neurotoxin type-A (BoNT/A), which blocks quantal acetylcholine (ACh) release at the neuromuscular junction (NMJ), has demonstrated its efficacy in the symptomatic treatment of blepharospasm. In 3.89% of patients treated for blepharospasm at Tenon Hospital, BoNT/A was no longer effective in relieving the patient's symptoms, and a partial upper myectomy of the Orbicularis oculi muscle was performed. We used surgical waste samples from 14 patients treated with repeated injections of either abobotulinumtoxinA (Dysport®) or incobotulinumtoxinA (Xeomin®). These muscle fragments were compared to others from 4 normal subjects, naïve of BoNT/A. The morphological study was performed blinded to the BoNT/A treatment and between treated and control samples. Neuromuscular specimens analyzed by confocal laser scanning microscopy, using fluorescent staining and immune-labeling of presynaptic proteins, revealed that the pattern of innervation (e.g., polyneuronal and convergent innervation), the muscle nicotinic ACh receptors (nAChRs), and the NMJs exhibited marked differences in BoNT/A-treated muscles (regardless of the toxin clinically used), with respect to controls. BoNT/A-treated junctions exhibited profuse polyneuronal innervation in which 2-6 axons innervated 74.84% of single muscle fibers, while 99.47% of control junctions were mono-innervated. Another new finding was the stable convergent innervation, in which several motor axons end onto the same endplate. Morphological signs of synapse elimination included the presence of retraction bulbs in axons and nerve terminals and a reduced extension of postsynaptic nAChRs. These outcomes suggest that synapse elimination is altered and raise questions on the origin and factors contributing to the plasticity changes observed and the functioning of NMJs.
Keywords: blepharospasm; botulinum type-A neurotoxin; convergent innervation; human Orbicularis oculi muscle; myectomy; nerve sprouting; neuromuscular junction; nicotinic acetylcholine receptors; polyneuronal innervation; skeletal muscle; synapse elimination.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the presented results.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

