Exploring Mycolactone-The Unique Causative Toxin of Buruli Ulcer: Biosynthetic, Synthetic Pathways, Biomarker for Diagnosis, and Therapeutic Potential
- PMID: 39728786
- PMCID: PMC11678992
- DOI: 10.3390/toxins16120528
Exploring Mycolactone-The Unique Causative Toxin of Buruli Ulcer: Biosynthetic, Synthetic Pathways, Biomarker for Diagnosis, and Therapeutic Potential
Abstract
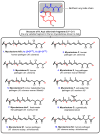
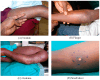
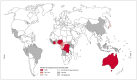
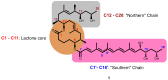
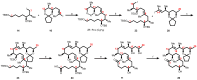
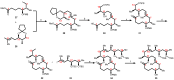
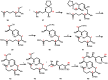
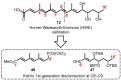
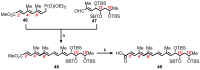
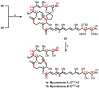
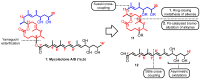
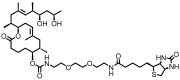
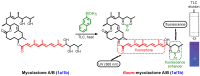
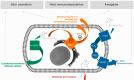
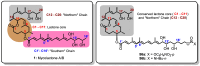
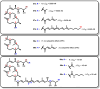
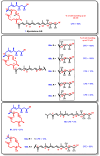
Mycolactone is a complex macrolide toxin produced by Mycobacterium ulcerans, the causative agent of Buruli ulcer. The aim of this paper is to review the chemistry, biosynthetic, and synthetic pathways of mycolactone A/B to help develop an understanding of the mode of action of these polyketides as well as their therapeutic potential. The synthetic work has largely been driven by the desire to afford researchers enough (≥100 mg) of the pure toxins for systematic biological studies toward understanding their very high biological activities. The review focuses on pioneering studies of Kishi which elaborate first-, second-, and third-generation approaches to the synthesis of mycolactones A/B. The three generations focused on the construction of the key intermediates required for the mycolactone synthesis. Synthesis of the first generation involves assignment of the relative and absolute stereochemistry of the mycolactones A and B. This was accomplished by employing a linear series of 17 chemical steps (1.3% overall yield) using the mycolactone core. The second generation significantly improved the first generation in three ways: (1) by optimizing the selection of protecting groups; (2) by removing needless protecting group adjustments; and (3) by enhancing the stereoselectivity and overall synthetic efficiency. Though the synthetic route to the mycolactone core was longer than the first generation, the overall yield was significantly higher (8.8%). The third-generation total synthesis was specifically aimed at an efficient, scalable, stereoselective, and shorter synthesis of mycolactone. The synthesis of the mycolactone core was achieved in 14 linear chemical steps with 19% overall yield. Furthermore, a modular synthetic approach where diverse analogues of mycolactone A/B were synthesized via a cascade of catalytic and/or asymmetric reactions as well as several Pd-catalyzed key steps coupled with hydroboration reactions were reviewed. In addition, the review discusses how mycolactone is employed in the diagnosis of Buruli ulcer with emphasis on detection methods of mass spectrometry, immunological assays, RNA aptamer techniques, and fluorescent-thin layer chromatography (f-TLC) methods as diagnostic tools. We examined studies of the structure-activity relationship (SAR) of various analogues of mycolactone. The paper highlights the multiple biological consequences associated with mycolactone such as skin ulceration, host immunomodulation, and analgesia. These effects are attributed to various proposed mechanisms of actions including Wiskott-Aldrich Syndrome protein (WASP)/neural Wiskott-Aldrich Syndrome protein (N-WASP) inhibition, Sec61 translocon inhibition, angiotensin II type 2 receptor (AT2R) inhibition, and inhibition of mTOR. The possible application of novel mycolactone analogues produced based on SAR investigations as therapeutic agents for the treatment of inflammatory disorders and inflammatory pain are discussed. Additionally, their therapeutic potential as anti-viral and anti-cancer agents have also been addressed.
Keywords: Buruli ulcer; analgesic and cytotoxic; aptamers; biomarker; immunosuppressive; mycolactone; toxin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

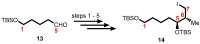
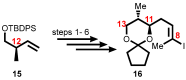
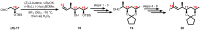
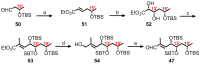
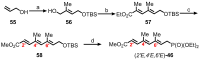
References
-
- WHO Global Buruli Ulcer Initiative (GBUI)) 2017. [(accessed on 11 November 2023)]. Available online: http://www.who.int/buruli/en/
-
- Leão S.C., Romano M.I., Garcia M.J. Bioinformatics in Tropical Disease Research: A Practical and Case-Study Approach [Internet] National Center for Biotechnology Information (US); Bethesda, MD, USA: 2007. Tuberculosis, Leprosy, and Other Mycobacterioses.
-
- Asiedu K., Raviglione M.C., Scherpbier R. Buruli Ulcer: Mycobacterium Ulcerans Infection. World Health Organization; Geneva, Switzerland: 2000.
-
- WHO The Global Health Observatory: Number of New Reported Cases of Buruli Ulcer. 2023. [(accessed on 28 May 2023)]. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/numbe....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

