Veratridine Induces Vasorelaxation in Mouse Cecocolic Mesenteric Arteries
- PMID: 39728791
- PMCID: PMC11679225
- DOI: 10.3390/toxins16120533
Veratridine Induces Vasorelaxation in Mouse Cecocolic Mesenteric Arteries
Abstract
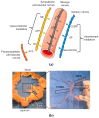
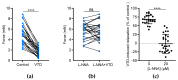
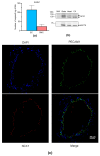
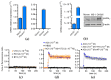
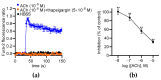
The vegetal alkaloid toxin veratridine (VTD) is a selective voltage-gated Na+ (NaV) channel activator, widely used as a pharmacological tool in vascular physiology. We have previously shown that NaV channels, expressed in arteries, contribute to vascular tone in mouse mesenteric arteries (MAs). Here, we aimed to better characterize the mechanisms of action of VTD using mouse cecocolic arteries (CAs), a model of resistance artery. Using wire myography, we found that VTD induced vasorelaxation in mouse CAs. This VTD-induced relaxation was insensitive to prazosin, an α1-adrenergic receptor antagonist, but abolished by atropine, a muscarinic receptor antagonist. Indeed, VTD-vasorelaxant effect was totally inhibited by the NaV channel blocker tetrodotoxin (0.3 µM), the NO synthase inhibitor L-NNA (20 µM), and low extracellular Na+ concentration (14.9 mM) and was partially blocked by the NCX1 antagonist SEA0400 (45.4% at 1 µM). Thus, we assumed that the VTD-induced vasorelaxation in CAs was due to acetylcholine release by parasympathetic neurons, which induced NO synthase activation mediated by the NCX1-Ca2+ entry mode in endothelial cells (ECs). We demonstrated NCX1 expression in ECs by RT-qPCR and immunohisto- and western immunolabelling. VTD did not induce an increase in intracellular Ca2+ ([Ca2+]i), while SEA0400 partially blocked acetylcholine-triggered [Ca2+]i elevations in Mile Sven 1 ECs. Altogether, these results illustrate that VTD activates NaV channels in parasympathetic neurons and then vasorelaxation in resistance arteries, which could explain arterial hypotension after VTD intoxication.
Keywords: Na+/Ca2+ exchanger (NCX); mesenteric and endothelial cell lines; mouse mesenteric arteries; myography; veratridine; voltage-gated Na+ channel.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Ulbricht W. Reviews of Physiology Biochemistry and Pharmacology, Volume 133. Springer; Berlin/Heidelberg, Germany: 1998. Effects of Veratridine on Sodium Currents and Fluxes; pp. 1–54. - PubMed
-
- Réthoré L., Park J., Montnach J., Nicolas S., Khoury J., Le Seac’h E., Mabrouk K., De Pomyers H., Tricoire-Leignel H., Mattei C., et al. Pharmacological Dissection of the Crosstalk between NaV and CaV Channels in GH3b6 Cells. Int. J. Mol. Sci. 2022;23:827. doi: 10.3390/ijms23020827. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

