Assessing the Potential Synergistic/Antagonistic Effects of Citrinin and Cannabidiol on SH-SY5Y, HepG2, HEK293 Cell Lines, and Human Lymphocytes
- PMID: 39728792
- PMCID: PMC11679033
- DOI: 10.3390/toxins16120534
Assessing the Potential Synergistic/Antagonistic Effects of Citrinin and Cannabidiol on SH-SY5Y, HepG2, HEK293 Cell Lines, and Human Lymphocytes
Abstract
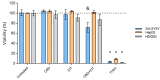
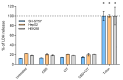
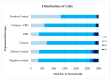
The increasing use of Cannabis sativa products for medicinal, dietary, and recreational purposes has raised concerns about mycotoxin contamination in cannabis and hemp. Mycotoxins persist in these products' post-processing, posing health risks via multiple exposure routes. This study investigated cytotoxic and genotoxic interactions between cannabidiol (CBD) and the mycotoxin citrinin (CIT) using human cell models: SH-SY5Y, HepG2, HEK293, and peripheral blood lymphocytes. IC50 values and membrane disruption were initially assessed, followed by an evaluation of genotoxicity in lymphocytes using the Comet Assay and Cytokinesis Blocked Micronucleus Cytome Assay. Obtained findings demonstrate that cell-type sensitivity varied across treatments, with combined CBD and CIT exposure exhibiting distinct interactions. Lactate dehydrogenase (LDH) release remained minimal, suggesting cytotoxicity did not stem from membrane disruption but likely involved intracellular pathways. In lymphocytes, CBD alone produced negligible cyto/genotoxic effects and weak antiproliferative responses, whereas CIT displayed clear toxic impacts. DNA damage indicates that CIT may induce genome instability through indirect mechanisms rather than direct DNA interaction, with evidence of potential aneuploidic effects from the CBMN Cyt Assay. Combined exposure led to a reduction in CIT-induced DNA and cytogenetic damage, suggesting CIT's potential interference with the beneficial properties of CBD. These results provide a foundation for further toxicological assessments and highlight the necessity of standardized mycotoxin monitoring in cannabis-derived products.
Keywords: Cannabis sativa; cell viability; contaminants; cytotoxicity; genotoxicity; hemp; moulds; mycotoxins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- International Agency for Research of Cancer (IARC) IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Volume 40 IARC P; Lyon, France: 1986.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

