Variations in Kojic Acid Production and Corn Infection Among Aspergillus flavus Isolates Suggest a Potential Role as a Virulence Factor
- PMID: 39728797
- PMCID: PMC11679525
- DOI: 10.3390/toxins16120539
Variations in Kojic Acid Production and Corn Infection Among Aspergillus flavus Isolates Suggest a Potential Role as a Virulence Factor
Abstract
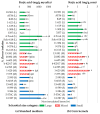
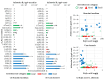
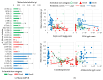
Kojic acid is a secondary metabolite with strong chelating and antioxidant properties produced by Aspergillus flavus and A. oryzae. Although antioxidants and chelators are important virulence factors for plant pathogens, the ecological role of kojic acid remains unclear. We previously observed a greater gene expression of antioxidants, especially kojic acid, by non-aflatoxigenic A. flavus when co-cultured with aflatoxigenic A. flavus. Aflatoxin production was also reduced. In this study, we investigated kojic acid production in 22 A. flavus isolates from Louisiana and compared them to four common A. flavus strains in liquid medium and on corn kernels. Corn kernel infection was assessed by quantifying the maize beta tubulin DNA content of the kernels using drop digital PCR (ddPCR). Maize beta tubulin DNA content decreased with increased corn kernel infection. Greater kojic acid production by A. flavus isolates coincided with greater levels of corn kernel infection. All isolates produced 60 and 700 times more kojic acid than aflatoxin and cyclopiazonic acid (a known virulence factor), respectively, which varied among sclerotial size categories. A. flavus strains with small sclerotia, which were rarely isolated from corn, produced the least kojic acid and infected corn kernels the least, while medium and large sclerotia strains-mainly isolated from corn-produced the most kojic acid and were more infectious. Non-aflatoxigenic isolates from Louisiana produced the most kojic acid. These results suggest that kojic acid is a potential virulence factor and may increase the pathogenic success of medium and large sclerotia-producing A. flavus, which could ultimately lead to more effective A. flavus biocontrol strains. Further studies are required to determine the effects that kojic acid has on the redox environment during corn infection and how the altered redox environment decreases aflatoxin production.
Keywords: aflatoxin; biological control; ddPCR; mycotoxins; pathogenicity; plant–fungal interactions; secondary metabolism.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Characterization and competitive ability of non-aflatoxigenic Aspergillus flavus isolated from the maize agro-ecosystem in Argentina as potential aflatoxin biocontrol agents.Int J Food Microbiol. 2018 Jul 20;277:58-63. doi: 10.1016/j.ijfoodmicro.2018.04.020. Epub 2018 Apr 13. Int J Food Microbiol. 2018. PMID: 29684766
-
Substrate depletion during formation of aflatoxin and kojic acid on corn inoculated with Aspergillus flavus.Mycopathologia. 1986 Feb;93(2):105-7. doi: 10.1007/BF00437741. Mycopathologia. 1986. PMID: 3086727
-
Comparison of soil and corn kernel Aspergillus flavus populations: evidence for niche specialization.Phytopathology. 2011 Aug;101(8):952-9. doi: 10.1094/PHYTO-09-10-0243. Phytopathology. 2011. PMID: 21405994
-
Molecular and aflatoxigenicity analyses of Aspergillus flavus isolates indigenous to grain corn in Malaysia; potentials for biological control.J Appl Microbiol. 2024 Jun 3;135(6):lxae145. doi: 10.1093/jambio/lxae145. J Appl Microbiol. 2024. PMID: 38877665
-
Phylogenetic studies and distinction of aflatoxin-producing Aspergillus species in section Flavi, Ochraceorosei and Nidulantes: A review.Gene. 2025 Feb 10;937:149151. doi: 10.1016/j.gene.2024.149151. Epub 2024 Dec 9. Gene. 2025. PMID: 39662646 Review.
References
-
- Richard J.L. Discovery of aflatoxins and significant historical features. Toxin Rev. 2008;27:171–201. doi: 10.1080/15569540802462040. - DOI
-
- Yu J., Hennessy D.A., Tack J., Wu F. Climate change will increase aflatoxin presence in US corn. Environ. Res. Lett. 2022;17:054017. doi: 10.1088/1748-9326/ac6435. - DOI
-
- Bandyopadhyay R., Ortega-Beltran A., Akande A., Mutegi C., Atehnkeng J., Kaptoge L., Senghor A.L., Adhikari B.N., Cotty P.J. Biological control of aflatoxins in Africa: Current status and potential challenges in the face of climate change. World Mycotoxin J. 2016;9:771–789. doi: 10.3920/WMJ2016.2130. - DOI
-
- Moral J., Garcia-Lopez M.T., Camiletti B.X., Jaime R., Michailides T.J., Bandyopadhyay R., Ortega-Beltran A. Present status and perspective on the future use of Aflatoxin Biocontrol products. Agronomy. 2020;10:491. doi: 10.3390/agronomy10040491. - DOI
-
- Cotty P.J., Antilla L., Wakelyn P.J. Competitive exclusion of aflatoxin producers: Farmer-driven research and development. In: Vincent C., Goettel M.S., Lazarovits G., editors. Biological Control: A Global Perspective. CAB International; Wallingford, UK: 2007. pp. 241–253.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

