Insights from a National Survey on Controlled Substance Diversion Practices in U.S. Hospital Pharmacies: Opportunities for Enhanced Surveillance and Compliance
- PMID: 39728848
- PMCID: PMC11676067
- DOI: 10.3390/pharmacy12060183
Insights from a National Survey on Controlled Substance Diversion Practices in U.S. Hospital Pharmacies: Opportunities for Enhanced Surveillance and Compliance
Abstract
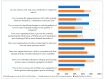
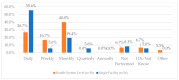
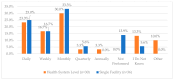
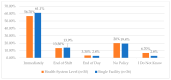
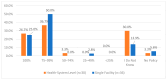
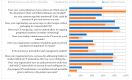
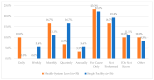
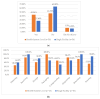
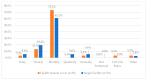
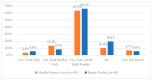
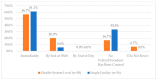
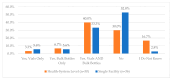
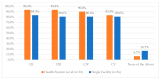
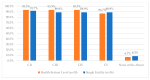
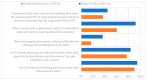
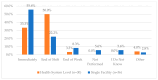
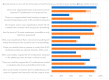
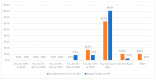
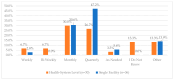
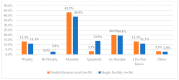
This study explored controlled substance (CS) diversion surveillance practices within hospital pharmacies across the United States. A survey with questions based on published CS diversion risk points was conducted in May 2024. A total of 66 participants from 31 states responded, with 54.5% from single facilities and the remaining from health systems. Most respondents were pharmacy directors, managers, or those in dedicated drug diversion roles. Over 70% have dedicated surveillance teams and use drug diversion software. Results highlight variation in practices, with larger institutions generally showing better compliance. Compliance in procurement and receiving was high for access measures; however, auditing of processes was lower. The lowest procurement compliance was in monitoring periodic automatic replacement (PAR) levels and validating orders with wholesalers. Storage practices showed high compliance in deploying cameras, but low compliance in monitoring them. Dispensing practices had high compliance for restricting CS in automated dispensing cabinets, but low incidence of witness verification during stocking. Waste and disposal practices were well-followed, but training on detecting potential signs of medication tampering was less common. The survey highlights that while strategies to prevent CS diversion exist, their implementation varies. Enhancing monitoring, auditing, and training is essential to strengthen diversion prevention efforts in hospital pharmacies.
Keywords: controlled substances; diversion; healthcare safety; hospital pharmacy; patient safety.
Conflict of interest statement
This work was supported by Becton, Dickinson, and Company (BD), San Diego, CA, USA. The sponsor assisted with study design, data collection, analysis and interpretation of the data, the writing of the report, and the decision to submit this article for publication as it is the sponsor’s standard policy to publish all human subjects research, regardless of outcomes. All authors are employees and shareholders of BD.
Figures
References
-
- United States Department of Justice Practitioner’s Manual: An Informational Outline of the Controlled Substances Act. [(accessed on 19 September 2024)];2023 Available online: https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-071)(EO-DEA226)_Practitio....
-
- Tortorici A., Turner B. Investigate and manage suspected drug diversion. Pharm. Purch. Prod. 2011;8:12–15.
LinkOut - more resources
Full Text Sources

