Pediatric Neuroimaging of Multiple Sclerosis and Neuroinflammatory Diseases
- PMID: 39728911
- PMCID: PMC11679236
- DOI: 10.3390/tomography10120149
Pediatric Neuroimaging of Multiple Sclerosis and Neuroinflammatory Diseases
Abstract
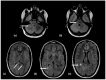
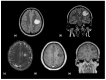
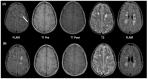
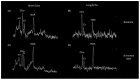
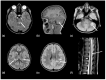
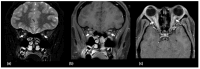
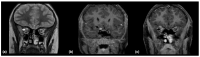
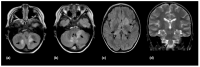
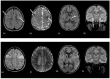
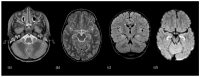
Using a pediatric-focused lens, this review article briefly summarizes the presentation of several demyelinating and neuroinflammatory diseases using conventional magnetic resonance imaging (MRI) sequences, such as T1-weighted with and without an exogenous gadolinium-based contrast agent, T2-weighted, and fluid-attenuated inversion recovery (FLAIR). These conventional sequences exploit the intrinsic properties of tissue to provide a distinct signal contrast that is useful for evaluating disease features and monitoring treatment responses in patients by characterizing lesion involvement in the central nervous system and tracking temporal features with blood-brain barrier disruption. Illustrative examples are presented for pediatric-onset multiple sclerosis and neuroinflammatory diseases. This work also highlights findings from advanced MRI techniques, often infrequently employed due to the challenges involved in acquisition, post-processing, and interpretation, and identifies the need for future studies to extract the unique information, such as alterations in neurochemistry, disruptions of structural organization, or atypical functional connectivity, that may be relevant for the diagnosis and management of disease.
Keywords: acute demyelinating encephalomyelitis; demyelination; magnetic resonance imaging; magnetic resonance spectroscopy; multiple sclerosis; myelin oligodendrocyte glycoprotein antibody disease; neuroinflammatory.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Uncommon Non-MS Demyelinating Disorders of the Central Nervous System.Curr Neurol Neurosci Rep. 2025 Jul 1;25(1):45. doi: 10.1007/s11910-025-01432-8. Curr Neurol Neurosci Rep. 2025. PMID: 40591029 Review.
-
Targeted magnetic resonance imaging (tMRI) of small changes in the T1 and spatial properties of normal or near normal appearing white and gray matter in disease of the brain using divided subtracted inversion recovery (dSIR) and divided reverse subtracted inversion recovery (drSIR) sequences.Quant Imaging Med Surg. 2023 Oct 1;13(10):7304-7337. doi: 10.21037/qims-23-232. Epub 2023 Aug 15. Quant Imaging Med Surg. 2023. PMID: 37869282 Free PMC article. Review.
-
MarkVCID cerebral small vessel consortium: II. Neuroimaging protocols.Alzheimers Dement. 2021 Apr;17(4):716-725. doi: 10.1002/alz.12216. Epub 2021 Jan 21. Alzheimers Dement. 2021. PMID: 33480157 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The effect of gadolinium-based contrast-agents on automated brain atrophy measurements by FreeSurfer in patients with multiple sclerosis.Eur Radiol. 2022 May;32(5):3576-3587. doi: 10.1007/s00330-021-08405-8. Epub 2022 Jan 3. Eur Radiol. 2022. PMID: 34978580 Free PMC article. Clinical Trial.
References
-
- Krupp L.B., Vieira M.C., Toledano H., Peneva D., Druyts E., Wu P., Boulos F.C. A Review of Available Treatments, Clinical Evidence, and Guidelines for Diagnosis and Treatment of Pediatric Multiple Sclerosis in the United States. J. Child. Neurol. 2019;34:612–620. doi: 10.1177/0883073819855592. - DOI - PubMed
-
- Prajjwal P., Marsool M.D.M., Natarajan B., Inban P., Gadam S., Sowndarya D., John J., Abbas R., Vaja H., Marsool M.B., et al. Juvenile multiple sclerosis: Addressing epidemiology, diagnosis, therapeutic, and prognostic updates along with cognitive dysfunction and quality of life. Ann. Med. Surg. 2023;85:4433–4441. doi: 10.1097/MS9.0000000000000930. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

