Establishment of a Real-Time Reverse Transcription Recombinase-Aided Isothermal Amplification (qRT-RAA) Assay for the Rapid Detection of Bovine Respiratory Syncytial Virus
- PMID: 39728929
- PMCID: PMC11680440
- DOI: 10.3390/vetsci11120589
Establishment of a Real-Time Reverse Transcription Recombinase-Aided Isothermal Amplification (qRT-RAA) Assay for the Rapid Detection of Bovine Respiratory Syncytial Virus
Abstract
Background: Bovine respiratory syncytial virus (BRSV) is a significant cause of bovine respiratory disease, resulting in significant losses to the cattle industry. For rapid detection of BRSV, a real-time recombinase-aided isothermal amplification assay (qRT-RAA) based on the F gene of BRSV was developed in this study.
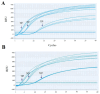
Results: The developed qRT-RAA assay showed good exponential amplification of the target fragment in 20 min at a constant temperature of 39 °C. And this assay displayed a high specificity for BRSV, without cross-reactions with Infectious Bovine Rhinotracheitis Virus (IBRV), Bovine Parainfluenza Virus Type 3 (BPIV3), Bovine Viral Diarrhea Virus (BVDV), and Bovine Coronavirus (BCoV). With the standard RNA of BRSV serving as a template, the limit of detection for qRT-RAA was 102 copies/μL. We examined ninety-seven clinical samples from cattle with respiratory disease using this method and determined a positive rate of 7.2% (7/97), consistent with results using the classical PCR method reported previously.
Conclusions: A qRT-RAA assay for BRSV detection was established in this study. The method is specific and sensitive and can be completed within 20 min at 39 °C. These works demonstrate that the generated qRT-RAA assay is an effective diagnostic tool for rapidly detecting BRSV in resource-limited settings, which may be applied for the clinical detection of BRSV.
Keywords: bovine respiratory syncytial virus; detection assay; qRT-RAA; sensitivity; specificity.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Establishment of a multiplex qPCR assay for the detection of pathogens associated with bovine respiratory disease complex.Front Vet Sci. 2025 Apr 22;12:1594488. doi: 10.3389/fvets.2025.1594488. eCollection 2025. Front Vet Sci. 2025. PMID: 40331218 Free PMC article.
-
Development of a nanoparticle-assisted PCR assay for detection of bovine respiratory syncytial virus.BMC Vet Res. 2019 Apr 11;15(1):110. doi: 10.1186/s12917-019-1858-0. BMC Vet Res. 2019. PMID: 30971257 Free PMC article.
-
Establishment and application of a rapid diagnostic method for BVDV and IBRV using recombinase polymerase amplification-lateral flow device.Front Vet Sci. 2024 Mar 27;11:1360504. doi: 10.3389/fvets.2024.1360504. eCollection 2024. Front Vet Sci. 2024. PMID: 38601910 Free PMC article.
-
Development of Rapid Isothermal Detection Methods Real-Time Fluorescence and Lateral Flow Reverse Transcription Recombinase-Aided Amplification Assay for Bovine Coronavirus.Transbound Emerg Dis. 2024 Feb 10;2024:7108960. doi: 10.1155/2024/7108960. eCollection 2024. Transbound Emerg Dis. 2024. PMID: 40303076 Free PMC article.
-
Review on bovine respiratory syncytial virus and bovine parainfluenza - usual suspects in bovine respiratory disease - a narrative review.BMC Vet Res. 2021 Jul 31;17(1):261. doi: 10.1186/s12917-021-02935-5. BMC Vet Res. 2021. PMID: 34332574 Free PMC article. Review.
References
-
- Bai H., Shi Q., Wang Y., Wang X. Research progress on detection methods of bovine respiratory syncytial virus. Chin. Vet. Sci. 2024;54:248–254.
-
- Liang X., Ning P., Li X., Bao X., Li H., Shi Y., Wang X., Guo X., Xu L. Establishment and application of a one-step real-time fluorescence quantitative PCR assay for bovine respiratory syncytial virus. Anim. Husb. Vet. Med. 2024;56:94–98.
-
- Jia S., Yao X., Yang Y., Niu C., Zhao Y., Zhang X., Pan R., Jiang X., Xiaobo S., Qiao X., et al. Isolation, identification, and phylogenetic analysis of subgroup iii strain of bovine respiratory syncytial virus contributed to outbreak of acute respiratory disease among cattle in Northeast China. Virulence. 2021;12:404–414. doi: 10.1080/21505594.2021.1872178. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

