The Venom of Vipera ammodytes ammodytes: Proteomics, Neurotoxic Effect and Neutralization by Antivenom
- PMID: 39728945
- PMCID: PMC11680118
- DOI: 10.3390/vetsci11120605
The Venom of Vipera ammodytes ammodytes: Proteomics, Neurotoxic Effect and Neutralization by Antivenom
Abstract
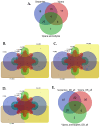
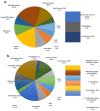
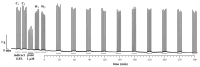
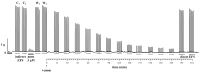
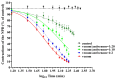
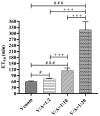
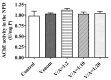
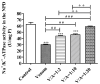
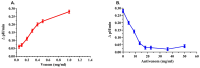
Deep proteomic analyses identified, in total, 159 master proteins (with 1% FDR and 2 unique peptides) from 26 protein families in the venom of Vipera ammodytes ammodytes (Vaa). Data are available via ProteomeXchange with the identifier PXD056495. The relative abundance of PLA2s is 11.60% of the crude venom, of which 4.35% are neurotoxic Ammodytoxins (Atxs). The neurotoxicity of the venom of Vaa and the neutralizing effect of the antivenom were tested on the neuromuscular preparation of the diaphragm (NPD) of rats. The activity of PLA2 in the venom of Vaa and its neutralization by the antivenom were determined under in vitro conditions. The Vaa venom leads to a progressive decrease in NPD contractions. We administered pre-incubated venom/antivenom mixtures at various ratios of 1:2, 1:10 and 1:20 (w/w) and observed the effects of these mixtures on NPD contractions. The results show that the mean effective time (ET50) for NPD contractions with the 1:20 mixture is highly significantly different (p < 0.001) from the ET50 for the venom and the ET50 for the 1:2 and 1:10 mixture ratios. We also found a highly significant (p < 0.001) reduction in Na+/K+-ATPase activity in the NPD under the influence of the venom. The reduction in the activity of this enzyme was reversible by the antivenom. Under in vitro conditions, we have achieved the complete neutralization of PLA2 by the antivenom. In conclusion, the antivenom abolished the venom-induced progressive decrease in NPD contractions in a concentration-dependent manner. Antivenom with approximately the same mass proportion almost completely restores Na+/K+-ATPase activity in the NPD and completely neutralizes the PLA2 activity of the venom in vitro.
Keywords: Na+/K+-ATPase; Vipera ammodytes ammodytes; antivenom; diaphragm; neurotoxicity; proteomics; venom.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

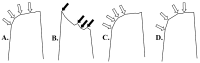
References
-
- Tomović L., Anđelković M., Krizmanić I., Ajtić R., Urošević A., Labus N., Simović A., Maričić M., Golubović A., Ćorović J., et al. Distribution of Three Vipera Species in the Republic of Serbia. Bull. Nat. Hist. Mus. 2019;12:217–242. doi: 10.5937/bnhmb1912217T. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

