Effects of Caffeic Acid Phenethyl Ester on Embryonic Development Through Regulation of Mitochondria and Endoplasmic Reticulum
- PMID: 39728965
- PMCID: PMC11680163
- DOI: 10.3390/vetsci11120625
Effects of Caffeic Acid Phenethyl Ester on Embryonic Development Through Regulation of Mitochondria and Endoplasmic Reticulum
Abstract
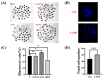
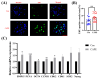
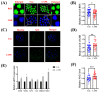
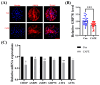
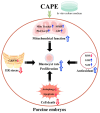
Caffeic acid phenethyl ester (CAPE) is one of the main active components of the natural medicine propolis, which has antioxidant, anti-tumor, and immunomodulatory activities. This study aimed to analyze the effects and underlying mechanisms of CAPE added to the medium of in vitro cultures on the developmental competence, mitochondria, and endoplasmic reticulum of porcine embryos. The results demonstrated that 1 nM of CAPE significantly improved the quality of porcine embryos, increased the rate of blastocyst formation, and enhanced the proliferation ability. It also enhanced mitochondrial function by increasing the level of mitochondrial membrane potential and expression of the mitochondrial biogenesis-related protein PPARgamma coactivator 1 alpha and beta (PGC1 alpha and beta), regulating mitochondrial biogenesis, and increasing adenosine triphosphate (ATP) content. In addition, CAPE alleviated oxidative and endoplasmic reticulum (ER) stress in embryos by decreasing ROS accumulation and increasing glutathione content, as well as elevating Nrf2 and reducing GRP78 (ER stress marker) expression levels. Moreover, CAPE reduced the levels of apoptosis and autophagy in the cultivated embryos. These results indicate that CAPE improves the quality and enhances the mitochondrial function of in vitro-produced porcine embryos by alleviating oxidative and ER stress.
Keywords: caffeic acid phenethyl ester; embryonic development; endoplasmic reticulum; mitochondrial function.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

