Substrate expansion of Geotrichum candidum alcohol dehydrogenase towards diaryl ketones by mutation
- PMID: 39729095
- PMCID: PMC11680648
- DOI: 10.1007/s00253-024-13375-0
Substrate expansion of Geotrichum candidum alcohol dehydrogenase towards diaryl ketones by mutation
Abstract
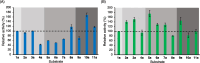
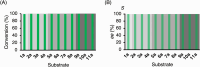
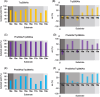
Chiral diaryl alcohols, such as (4-chlorophenyl)(pyridin-2-yl)methanol, are important intermediates for pharmaceutical synthesis. However, using alcohol dehydrogenases (ADHs) in the asymmetric reduction of diaryl ketones to produce the corresponding alcohols is challenging due to steric hindrance in the substrate binding pockets of the enzymes. In this study, the steric hindrance of the ADH from Geotrichum candidum NBRC 4597 (G. candidum acetophenone reductase, GcAPRD) was eliminated by simultaneous site-directed mutagenesis of Phe56 (in the large pocket) and Trp288 (in the small pocket). As a result, two double mutants, Phe56Ile/Trp288Ala, and Phe56Ala/Trp288Ala, exhibited much higher specific activities towards 2-(4'-chlorobenzoyl)pyridine (4.5 μmol/min/mg and 3.4 μmol/min/mg, respectively) than the wild type (< 0.2 μmol/min/mg). In whole-cell-catalyzed asymmetric reductions of diaryl ketones, Phe56Ile/Trp288Ala significantly increased the isolated yields, which were over 90% for the reactions of most of the tested substrates. Regarding enantioselectivity, Phe56Ile/Trp288Ala and Phe56Ala/Trp288Ala, and Trp288Ala generally exhibited similar selectivity to produce (R)-alcohols with up to 97% ee. KEY POINTS: • Phe56 in Geotrichum reductase (GcAPRD) was mutated to eliminate steric hindrance. • Mutation at Phe56 increased enzymatic activity and expanded substrate specificity. • Phe56Ile/Trp288Ala showed high activity and (R)-selectivity towards diaryl ketones.
Keywords: Alcohol dehydrogenase; Asymmetric reduction; Chiral diaryl alcohol; Diaryl ketone; Enzyme engineering; Site-directed mutagenesis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures

Similar articles
-
Reversible control of enantioselectivity by the length of ketone substituent in biocatalytic reduction.Appl Microbiol Biotechnol. 2019 Dec;103(23-24):9529-9541. doi: 10.1007/s00253-019-10206-5. Epub 2019 Nov 13. Appl Microbiol Biotechnol. 2019. PMID: 31720775
-
Structural basis for a highly (S)-enantioselective reductase towards aliphatic ketones with only one carbon difference between side chain.Appl Microbiol Biotechnol. 2019 Dec;103(23-24):9543-9553. doi: 10.1007/s00253-019-10093-w. Epub 2019 Nov 15. Appl Microbiol Biotechnol. 2019. PMID: 31482280
-
Structural Insight into Enantioselective Inversion of an Alcohol Dehydrogenase Reveals a "Polar Gate" in Stereorecognition of Diaryl Ketones.J Am Chem Soc. 2018 Oct 3;140(39):12645-12654. doi: 10.1021/jacs.8b08640. Epub 2018 Sep 24. J Am Chem Soc. 2018. PMID: 30247889
-
Impact and relevance of alcohol dehydrogenase enantioselectivities on biotechnological applications.Appl Microbiol Biotechnol. 2020 Apr;104(7):2897-2909. doi: 10.1007/s00253-020-10440-2. Epub 2020 Feb 15. Appl Microbiol Biotechnol. 2020. PMID: 32060695 Review.
-
New alcohol dehydrogenases for the synthesis of chiral compounds.Adv Biochem Eng Biotechnol. 1997;58:145-84. doi: 10.1007/BFb0103304. Adv Biochem Eng Biotechnol. 1997. PMID: 9103913 Review.
References
-
- Bagchi B (2013) The hydrophobic effect. In: Water in biological and chemical processes: from structure and dynamics to function. Cambridge University Press, 215–242
-
- Bsharat O, Musa MM, Vieille C, Oladepo SA, Takahashi M, Hamdan SM (2017) Asymmetric reduction of substituted 2-tetralones by Thermoanaerobacter pseudoethanolicus secondary alcohol dehydrogenase. ChemCatChem 9:1487–1493. 10.1002/cctc.201601618
-
- Chen Q, Guo M, Bi Y, Qu G, Sun Z, Wang Y, Luo G (2021) Whole-cell biocatalytic synthesis of S -(4-chlorophenyl)-(pyridin-2-yl) methanol in a liquid–liquid biphasic microreaction system. Bioresour Technol 330:125022. 10.1016/j.biortech.2021.125022 - PubMed
-
- Ensari Y, Dhoke GV, Davari MD, Ruff AJ, Schwaneberg U (2018) A comparative reengineering study of cpADH5 through iterative and simultaneous multisite saturation mutagenesis. ChemBioChem 19:1563–1569. 10.1002/cbic.201800159 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

