A stratified treatment algorithm in psychiatry: a program on stratified pharmacogenomics in severe mental illness (Psych-STRATA): concept, objectives and methodologies of a multidisciplinary project funded by Horizon Europe
- PMID: 39729102
- PMCID: PMC12271288
- DOI: 10.1007/s00406-024-01944-3
A stratified treatment algorithm in psychiatry: a program on stratified pharmacogenomics in severe mental illness (Psych-STRATA): concept, objectives and methodologies of a multidisciplinary project funded by Horizon Europe
Abstract
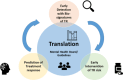
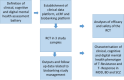
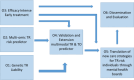
Schizophrenia (SCZ), bipolar (BD) and major depression disorder (MDD) are severe psychiatric disorders that are challenging to treat, often leading to treatment resistance (TR). It is crucial to develop effective methods to identify and treat patients at risk of TR at an early stage in a personalized manner, considering their biological basis, their clinical and psychosocial characteristics. Effective translation of theoretical knowledge into clinical practice is essential for achieving this goal. The Psych-STRATA consortium addresses this research gap through a seven-step approach. First, transdiagnostic biosignatures of SCZ, BD and MDD are identified by GWAS and multi-modal omics signatures associated with treatment outcome and TR (steps 1 and 2). In a next step (step 3), a randomized controlled intervention study is conducted to test the efficacy and safety of an early intensified pharmacological treatment. Following this RCT, a combined clinical and omics-based algorithm will be developed to estimate the risk for TR. This algorithm-based tool will be designed for early detection and management of TR (step 4). This algorithm will then be implemented into a framework of shared treatment decision-making with a novel mental health board (step 5). The final focus of the project is based on patient empowerment, dissemination and education (step 6) as well as the development of a software for fast, effective and individualized treatment decisions (step 7). The project has the potential to change the current trial and error treatment approach towards an evidence-based individualized treatment setting that takes TR risk into account at an early stage.
Keywords: Depression schizophrenia; Early detection; Early treatment; Precision psychiatry; Treatment resistance.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: BTB received honoraria from Angelini, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol-Meyers Squibb, Janssen, LivaNova, Lundbeck, Medscape, Neurotorium, Novartis, Otsuka, Pfizer, Recordati, Roche, Rovi, Sanofi, Servier, and Teva. AS is, or has been a consultant to, or has received honoraria or grants unrelated to the present work from Abbott, Abbvie, Angelini, Astra Zeneca, Clinical Data, Boehringer, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Innovapharma, Italfarmaco, Janssen, Lundbeck, Naurex, Pfizer, Polifarma, Sanofi, Servier, and Taliaz. AM received advisory board or consultant fees from Angelini, Pierre Fabre, Rovi Pharma, and Boehringer Ingelheim outside the submitted work. OH has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by Alkermes, Angellini, Autifony, Biogen, Boehringer Ingelheim, Delix, Eli Lilly, Elysium, Heptares, Global Medical Education, Invicro, Janssen, Karuna, Lundbeck, Merck, Neumora, Neurocrine, Ontrack/Pangea, Otsuka, Sunovion, Teva, Recordati, Roche, Rovi, and Viatris/Mylan. He was previously a part-time employee of Lundbeck A/v. HF received grants from Abbvie, UCB, and Neuway Pharma. PR received honoraria as a consultant for lectures from Angelini, Janssen, Lundbeck, and Otsuka. Furthermore, PR is on Angelini’s advisory board. OAA is a consultant to Cortechs.ai and Precision Health.ai, and has received speaker’s honoraria from Janssen, Lundbeck, Sunovion, and Otsuka. LVK has been a consultant for Lundbeck and Teva within the preceding three years. AH has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by Boehringer Ingelheim, Gedeon Richter, Janssen, Lundbeck, Newron Pharmaceuticals, and Rovi. AV received advisory board, lecture, or consulting fees outside the present work from Angelini, Innova Pharma-Recordati, Janssen Pharmaceuticals, Lundbeck, Otsuka, and Pfizer. CF was a speaker for Janssen. MM received honoraria from Angelini, Lundbeck, Johnson & Johnson, and Fidia Farmaceutici. UE-P reports consultancy for Boehringer Ingelheim and speaker honorarium from Angelini Pharma. AR has received honoraria for lectures and/or advisory boards from Janssen, Boehringer Ingelheim, COMPASS, SAGE/Biogen, LivaNova, Medice, Shire/Takeda, MSD, and cyclerion. In addition, he has received research grants from Medice and Janssen. KDP and AA are employed by NovaMechanics, a cheminformatics company. JTRW was supported by a collaborative research grant from Takeda Pharmaceuticals Ltd. for a project unrelated to work presented here. AFP and JTRW also received grants from Akrivia Health for a project unrelated to this research. EV has received grants and served as a consultant, advisor, or CME speaker for AB-Biotics, AbbVie, Adamed, Alcediag, Angelini, Biogen, Beckley-Psytech, Biohaven, Boehringer Ingelheim, Celon Pharma, Compass, Dainippon Sumitomo Pharma, Ethypharm, Ferrer, Gedeon Richter, GH Research, Glaxo-Smith Kline, HMNC, Idorsia, Johnson & Johnson, Lundbeck, Luye Pharma, Medincell, Merck, Newron, Novartis, Orion Corporation, Organon, Otsuka, Roche, Rovi, Sage, Sanofi-Aventis, Sunovion, Takeda, Teva, and Viatris outside the submitted work. LI has received CME-related honoraria and/or travel support from Angelini, Casen Recordati, Exeltis, Janssen-Cilag, Lundbeck, Rovi, Rubió, and Takeda outside the submitted work. PF served as a consultant or CME speaker for Idorsia, Lundbeck, and Janssen. AR has received honoraria from and/or serves on advisory boards for Medice, Shire/Takeda, SAGE/Biogen, Janssen, Boehringer Ingelheim, and cyclerion. MW serves as a consultant, speaker, and received funding for research from Janssen, Alkermes, Lundbeck, Teva, Newron, Dexcel, MSD, and Minerva. SB received advisory board, lecture, or consulting fees outside the present work from Angelini, Janssen Pharmaceuticals, Lundbeck, and Otsuka. ML has received lecture honoraria from Lundbeck pharmaceuticals. CB, IW, JL, SEF, KL, JE, DW, CS, COP, PN, PB, M-CP, MA, TDK, MML, and SE declare no conflict of interest. RR received an unrestricted research grant and speaker honoraria from Aristo Pharma. MA received research grants from Berlin University Alliance, Volkswagen Stiftung, Stiftung für Analytische Psychiatrie, and Gilead as well as speaker or consultation honoraria or travel reimbursements from Johnson & Johnson, Aristo Pharma, Gilead, and Neuraxpharm. BMM has a secondary appointment as head of precision psychiatry with HMNC Brain Health. CM has received honoraria as a consultant and/or advisor for lectures from Angelini, Compass, Esteve, Exeltis Janssen, Lundbeck, Neuraxpharm, Nuvelution, Otsuka, Pfizer, Servier, and Sunovion outside the submitted work. CMDC has received honoraria and/or travel support from Angelini, Janssen, and Viatris not related to the submitted work. AHY received paid lectures and advisory boards from Flow Neuroscience, Novartis, Roche, Janssen, Takeda, Noemapharma, Compass, Astrazenaca, Boehringer Ingelheim, Eli Lilly, LivaNova, Lundbeck, Sunovion, Servier, Livanova, Janssen, Allegan, Bionomics, Sumitomo Dainippon Pharma, Sage, and Neurocentrx. KOS received honoraria from Janssen and Lundbeck as well as research funding from Janssen, Lundbeck, and Gilead. Ethical approval: The RCT in step 3 has been planned in accordance with the Helsinki Declaration and will be conducted in this way. In addition, ethical approvals have either already been granted or are pending but have been applied for: Australia: not yet approved. Austria: SZ: 2023–506602-39–00, BD: 2023–506605-19–00, MDD: 2023–506617-21–00. All approved by the ethical committee of the Medical University of Innsbruck. Germany: SZ: 2023–506602-39–00 approved by Ethik-Kommission des Landes Berlin, BD: 2023–506605-19–00 approved by Ethik-Kommission der Ärztekammer Westfalen-Lippe und der Universität Münster, MDD: 2023–506617-21–00 approved by Ethik-Kommission der Ärztekammer Nordrhein. Greece: SZ: 2023–506602-39–00, BD: 2023–506605-19–00, MDD: 2023–506617-21–00 Yet to be submitted; site was added later. Israel: merged protocol 0293–23-SMC, approved by the ethical committee of The Chaim Sheba Medical Center. Italy: SZ: 2023–506602-3900, BD: 2023–506605-19–00, MDD: 2023–506617-21–00. All approved by Comitato Etico Territoriale (CET) delle Marche. Poland: SZ: 2023–506602-3900, BD: 2023–506605-19–00, MDD: 2023–506617-21–00 Yet to be submitted; site was added later. Spain: SZ: 2023–506602-39–00, BD: 2023–506605-19–00, MDD: 2023–506617-21–00. All approved by CEIm del Hospital Clínic de Barcelona. United Kingdom: SZ: 2023–506602-39–00 & 23/EM/0266 approved by East Midlands—Nottingham 2 Research Ethics Committee, BD: 2023–506605-19–00 (not yet approved), MDD: 2023–506617-21–00 (not yet approved).
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous