Novel isothermal nucleic acid amplification method for detecting malaria parasites
- PMID: 39729108
- PMCID: PMC11680615
- DOI: 10.1007/s00253-024-13357-2
Novel isothermal nucleic acid amplification method for detecting malaria parasites
Abstract
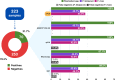
Malaria, a parasitic disease caused by Plasmodium spp. and transmitted by Anopheles mosquitoes, remains a major global health issue, with an estimated 249 million cases and 608,000 deaths in 2022. Rapid and accurate diagnosis and treatment are crucial for malaria control and elimination. However, limited access to sensitive molecular tests means that microscopic examination and rapid diagnostic tests (RDT) are the most used methods in endemic areas, despite their lower diagnostic accuracy. Therefore, there is a need for developing sensitive, simple, accurate, and rapid diagnostic tools suitable for field conditions. Herein, we aimed to explore the potential of the enzymatic recombinase amplification assay (ERA® Technology) as a remote laboratory test by evaluating and validating the GENEYE® ERA Plasmodium detection kit in Brazilian endemic areas. A cross-sectional cohort study was conducted between June and August of 2023 in the Brazilian Amazon. The study enrolled 323 participants residing in three malaria-affected regions: Cruzeiro do Sul and Mâncio Lima (Acre State) and Guajará (Amazonas State). The participants were tested for malaria by microscopy, rapid diagnostic tests (RDT), nested PCR (nPCR), quantitative real-time PCR (qPCR), and ERA. The sensitivity, specificity, and predictive values were assessed using nPCR as a gold standard. Plasmodium prevalence was 21.7%, 18.8%, 19.2%, 21.7%, and 21.7% by nPCR, microscopy, RDT, qPCR, and ERA respectively. Using nPCR as the standard, qPCR, and ERA showed a sensitivity of 100%. In comparison, microscopy and RDT showed a sensitivity of 87.1% and 88.6%, a negative predictive value (NPV) of 96.56 and 96.93, and kappa values of 0.91 and 0.92, respectively. For Plasmodium falciparum, the sensitivity of qPCR and ERA was 100% while the sensitivity of microscopy and RDT was 96.9% and 93.7%, and the NPV was 99.66 and 99.32, respectively. For Plasmodium vivax, only ERA showed the same sensitivity of nPCR. The sensitivity, NPV, and kappa values were 78.85%, 97.27, and 0.87 for qPCR and microscopy, and 84.21%, 97.94, and 0.9 for RDT. The data presented here show that the GENEYE® ERA Plasmodium detection kit offers a promising alternative to traditional malaria diagnostic methods. Its high sensitivity, specificity, fast processing time, and operational simplicity position it as a valuable point-of-care diagnostic tool, particularly in resource-limited and remote malaria-endemic areas. KEY POINTS: • GENEYE® ERA kit detects Plasmodium in under 25 min, no DNA purification needed. • The kit matches or exceeds the compared methods in sensitivity and specificity. • The kit is suitable for accurate testing in low-infrastructure, point-of-care settings.
Keywords: Plasmodium detection; Brazilian Amazon; Isothermal nucleic acid amplification; Malaria; Point-of-care; Rapid diagnostic tests.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The Ethics Committee of the Oswaldo Cruz Foundation of the Brazilian Ministry of Health (approval number CEP-FIOCRUZ CAAE 46084015.1.0000.5248) approved the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study. Conflict of interest: The authors declare no competing interests.
Figures

References
-
- Brasil (2020) Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Imunização e Doenças Transmissíveis: Guia de tratamento da malária no Brasil. Ministério da Saúde. http://bvsms.saude.gov.br/bvs/publicacoes/guia_tratamento_malaria_brasil.... Accessed 29 June 2024
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

