Prognostic and functional role of the nuclear export receptor 1 (XPO1) in gastrointestinal cancers: a potential novel target?
- PMID: 39729162
- PMCID: PMC11680630
- DOI: 10.1007/s11033-024-10169-5
Prognostic and functional role of the nuclear export receptor 1 (XPO1) in gastrointestinal cancers: a potential novel target?
Abstract
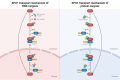
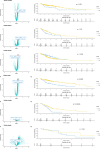
In the last decades the survival of metastatic gastrointestinal (GI) cancer patients could have been significantly extended due to the introduction of targeted- and immunotherapy. However, only the minority of patients will experience long-lasting survival. Hence, novel therapeutics are clearly necessary for GI cancer patients. Molecular high-throughput profiling techniques have revealed potential novel targetable molecular alterations, emphasizing the necessity for tailored therapeutic approaches. Nuclear export proteins, particularly Exportin-1 (XPO1), have emerged as promising targets in cancer therapy due to their crucial role in cellular homeostasis and regulation of key cellular functions. Dysregulation of XPO1-mediated nuclear export leads to the functional loss of tumor suppressors and pro-apoptotic factors, facilitating cancer progression. Selinexor, a XPO1 inhibitor, has shown promising activity in preclinical and clinical studies, particularly in hematological malignancies. However, its efficacy in GI cancers remains underexplored. This review aims to elucidate the functional and pathophysiological role of XPO1 in GI cancers. Despite the potential of XPO1 inhibitors in suppressing cell proliferation and inducing apoptosis, comprehensive molecular landscape data and validation of selective inhibitors in GI cancers are lacking. Targeting XPO1 presents a significant therapeutic potential for the treatment of GI cancer patients. Further research is necessary to fully elucidate the molecular landscape according to XPO1 expression in GI tumors and to validate the efficacy of selective XPO1 inhibitors. These efforts are expected to contribute to the development of more effective and personalized therapeutic strategies for GI cancer patients.
Keywords: Cancer therapy; Gastrointestinal cancers; Nuclear export proteins; SINE; Selinexor; XPO1; XPO1 inhibitor.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval: Not applicable. Consent to participate: Not applicable. Consent to publish: Not applicable.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

