Retinoblastoma and beyond: pediatric orbital mass lesions
- PMID: 39729290
- PMCID: PMC11893699
- DOI: 10.1007/s00234-024-03517-6
Retinoblastoma and beyond: pediatric orbital mass lesions
Abstract
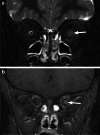
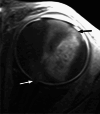
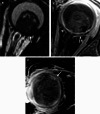
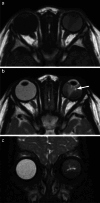
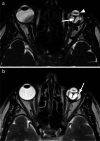
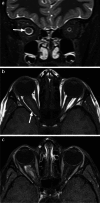
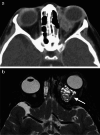
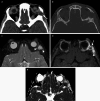
Various space occupying lesions can arise in the orbit, ranging from developmental anomalies to malignancies, and many of the diseases occurring in children are different from the pathologies in the adult population. As the clinical presentation is frequently nonspecific, radiologic evaluation is essential for lesion detection and characterization as well as patient management. While orbital masses may in some cases involve multiple compartments, a simple compartmental approach is the key for the diagnosis on imaging studies, and MRI is the modality of choice. This pictorial review presents the most common and characteristic non-emergent pediatric orbital lesions, stressing their MRI and CT appearances, including specific differentiating features. The lesions are subdivided into 4 compartments: intraocular, intraconal, extraconal, and orbital walls. Retinoblastoma, Coats disease and persistent fetal vasculature; optic pathway glioma and lymphovascular malformations; rhabdomyosarcoma, infantile hemangioma, neurofibroma and lymphoma; neuroblastoma, leukemia/myeloid sarcoma, Langerhans cell histiocytosis and dermoid are reviewed in their respective compartments.
Keywords: Dermoid; Infantile hemangioma; Langerhans cell histiocytosis; Neuroblastoma; Pseudoretinoblastoma; Retinoblastoma; Rhabdomyosarcoma; Venolymphatic malformations.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Ethical approval is not required for this review article, according to the institutional Ethics Committee. Informed consent: Informed consent was not necessary as the images used in this review article contain no identifying information. Competing interests: The authors have no competing interests to declare that are relevant to the content of this article. Conflict of interest: We declare that we have no conflict of interest.
Figures


























References
-
- Joseph AK, Guerin JB, Eckel LJ, Dalvin LA, Keating GF, Liebo GB, Benson JC, Brinjikji W, Laack NN, Silvera VM (2022) Imaging findings of pediatric orbital masses and tumor mimics. Radiographics 42:880–897. 10.1148/rg.210116 - PubMed
-
- Morris JA, Chiu SS, Rahim TA, Schaffner SR, Bajaj M (2022) An eye for eyes: Pediatric ophthalmologic diseases on MRI. Neurographics 12:103–116
-
- Koc G, Doganay S, Ciraci S, Dogan MS, Karakukcu M, Evereklioglu C, Coskun A (2017) Imaging evaluation of pediatric orbital pathologies. J Ayub Med Coll Abbottabad 29:523–528 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

