Estimated Vaccine Effectiveness for Pediatric Patients With Severe Influenza, 2015-2020
- PMID: 39729317
- PMCID: PMC11681373
- DOI: 10.1001/jamanetworkopen.2024.52512
Estimated Vaccine Effectiveness for Pediatric Patients With Severe Influenza, 2015-2020
Abstract
Importance: Increasing the understanding of vaccine effectiveness (VE) against levels of severe influenza in children could help increase uptake of influenza vaccination and strengthen vaccine policies globally.
Objective: To investigate VE in children by severity of influenza illness.
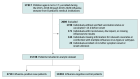
Design, setting, and participants: This case-control study with a test-negative design used data from 8 participating medical centers located in geographically different US states in the New Vaccine Surveillance Network from November 6, 2015, through April 8, 2020. Participants included children 6 months through 17 years of age who were hospitalized or presented to an emergency department (ED) with acute respiratory illness.
Exposures: Receipt of at least 1 dose of the current season's influenza vaccine.
Main outcomes and measures: Demographic and clinical characteristics of patients presenting to the hospital or ED with or without influenza were recorded and grouped by influenza vaccination status. Estimated VE against severe influenza illness was calculated using multiple measures to capture illness severity. Data were analyzed between June 1, 2022, and September 30, 2023.
Results: Among 15 728 children presenting for care with acute respiratory illness (8708 [55.4%] male; 13 450 [85.5%] 6 months to 8 years of age and 2278 [14.5%] 9-17 years of age), 2710 (17.2%) had positive influenza tests and 13 018 (82.8%) had negative influenza tests (controls). Of the influenza test-positive cases, 1676 children (61.8%) had an ED visit, 896 children (33.1%) required hospitalization for noncritical influenza, and 138 children (5.1%) required hospitalization for critical influenza. About half (7779 [49.5%]) of the children (both influenza test positive and test negative) were vaccinated. Receiving at least 1 influenza vaccine dose was estimated to have a VE of 55.7% (95% CI, 51.6%-59.6%) for preventing influenza-associated ED visits or hospitalizations among children of all ages. The estimated VE was similar across severity levels: 52.8% (95% CI, 46.6%-58.3%) for ED visits, 52.3% (95% CI, 44.8%-58.8%) for noncritical hospitalization, and 50.4% (95% CI, 29.7%-65.3%) for critical hospitalization.
Conclusions and relevance: Findings from this case-control study with a test-negative design involving children with a spectrum of influenza severity suggest that influenza vaccination protects children against all levels of severe influenza illness.
Conflict of interest statement
Figures
References
-
- Centers for Disease Control and Prevention . Flu and children at higher risk. Accessed October 27, 2022. https://www.cdc.gov/flu/highrisk/children.html?CDC_AAref_Val=https://www...
-
- US Department of Health and Human Services Office of Disease Prevention and Health Promotion . Increase the proportion of people who get the flu vaccine every year—IID-09. Accessed January 9, 2024. https://health.gov/healthypeople/objectives-and-data/browse-objectives/v...
-
- Centers for Disease Control and Prevention . Flu vaccination coverage, United States, 2021-22 influenza season. Accessed October 27, 2022. https://www.cdc.gov/fluvaxview/coverage-by-season/2021-2022.html?CDC_AAr...
-
- Centers for Disease Control and Prevention . Influenza vaccination coverage, children 6 months through 17 years. Accessed February 15, 2024. https://www.cdc.gov/fluvaxview/dashboard/children-vaccination-coverage.h...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous