MicroMetaSense: Coupling Plasmonic Metasurfaces with Fluorescence for Enhanced Detection of Microplastics in Real Samples
- PMID: 39729532
- PMCID: PMC11877509
- DOI: 10.1021/acssensors.4c02070
MicroMetaSense: Coupling Plasmonic Metasurfaces with Fluorescence for Enhanced Detection of Microplastics in Real Samples
Abstract
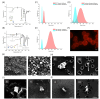
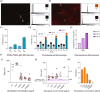
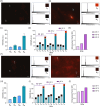
Diverse analytical techniques are employed to scrutinize microplastics (MPs)─pervasive at hazardous concentrations across diverse sources ranging from water reservoirs to consumable substances. The limitations inherent in existing methods, such as their diminished detection capacities, render them inadequate for analyzing MPs of diminutive dimensions (microplastics: 1-5 μm; nanoplastics: < 1 μm). Consequently, there is an imperative need to devise methodologies that afford improved sensitivity and lower detection limits for analyzing these pollutants. In this study, we introduce a holistic strategy, i.e., MicroMetaSense, reliant on a metal-enhanced fluorescence (MEF) phenomenon in detecting a myriad size and types of MPs (i.e., poly(methyl methacrylate) (PMMA) and poly(ethylene terephthalate) (PET)) down to 183-205 fg, as well as validated the system with real samples (tap and lake) and artificial ocean samples as a real-world scenario. To obtain precise size distribution in nanometer scale, MPs are initially processed with an ultrafiltration on-a-chip method, and subsequently, the MPs stained with Nile Red dye are subjected to meticulous analysis under a fluorescence microscope, utilizing both a conventional method (glass substrate) and the MicroMetaSense platform. Our approach employs a metasurface to augment fluorescence signals, leveraging the MEF phenomenon, and it demonstrates an enhancement rate of 36.56-fold in detecting MPs compared to the standardized protocols. This low-cost ($2), time-saving (under 30 min), and highly sensitive (183-205 femtogram) strategy presents a promising method for precise size distribution and notable improvements in detection efficacy not only for laboratory samples but also in real environmental samples; hence, signifying a pivotal advancement in conventional methodologies in MP detection.
Keywords: fluorescence microscopy; metal-enhanced fluorescence; microplastics; nanoplastics; plasmonic metasurfaces.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Tudor V. C.; Marin A.; Vasca D. Z.; Micu M. M.; Smedescu D. I. The Influence of the Plastic Bags on the Environment. Mater. Plast. 2018, 55 (4), 595. 10.37358/MP.18.4.5081. - DOI
-
- Ncube L. K.; Ude A. U.; Ogunmuyiwa E. N.; Zulkifli R.; Beas I. N. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6 (1), 12. 10.3390/recycling6010012. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

