Compartmentalizing Donor-Acceptor Stenhouse Adducts for Structure-Property Relationship Analysis
- PMID: 39729546
- PMCID: PMC11726581
- DOI: 10.1021/jacs.4c14198
Compartmentalizing Donor-Acceptor Stenhouse Adducts for Structure-Property Relationship Analysis
Abstract
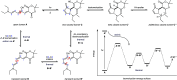
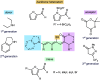
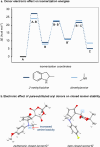
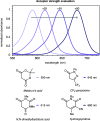
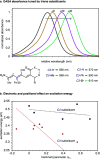
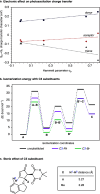
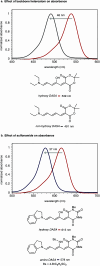
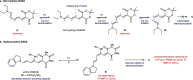
The development of photoswitches that absorb low energy light is of notable interest due to the growing demand for smart materials and therapeutics necessitating benign stimuli. Donor-acceptor Stenhouse adducts (DASAs) are molecular photoswitches that respond to light in the visible to near-infrared spectrum. As a result of their modular assembly, DASAs can be modified at the donor, acceptor, triene, and backbone heteroatom molecular compartments for the tuning of optical and photoswitching properties. This Perspective focuses on the electronic and steric contributions at each compartment and how they influence photophysical properties through the adjustment of the isomerization energetic landscape. An emphasis on current synthetic strategies and their limitations highlights opportunities for DASA architecture, and thus photophysical property expansion.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





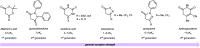


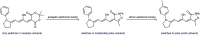
References
-
- Boelke J.; Hecht S. Designing Molecular Photoswitches For Soft Materials Applications. Adv. Opt. Mater. 2019, 7, 1900404 10.1002/adom.201900404. - DOI
-
- Abdollahi A.; Roghani-Mamaqani H.; Razavi B. Stimuli-Chromism Of Photoswitches In Smart Polymers: Recent Advances And Applications. Prog. Polym. Sci. 2019, 98, 101149 10.1016/j.progpolymsci.2019.101149. - DOI
-
- Andreasson J.; Pischel U. Light-Stimulated Molecular And Supramolecular Systems For Information Processing And Beyond. Coord. Chem. Rev. 2021, 429, 213695 10.1016/j.ccr.2020.213695. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

