DNMT3b-mediated CpA methylation facilitates REST binding and gene silencing and exacerbates hippocampal demyelination in diabetic mice
- PMID: 39730060
- PMCID: PMC11910331
- DOI: 10.1016/j.jbc.2024.108137
DNMT3b-mediated CpA methylation facilitates REST binding and gene silencing and exacerbates hippocampal demyelination in diabetic mice
Abstract
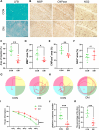
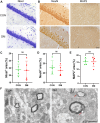
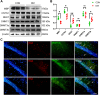
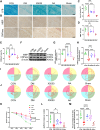
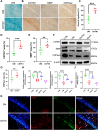
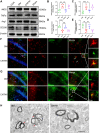
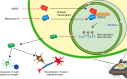
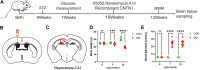
The remyelination process within the diabetes mellitus (DM) brain is inhibited, and dynamic interactions between DNA methylation and transcription factors are critical for this process. Repressor element-1 silencing transcription factor (REST) is a major regulator of oligodendrocyte differentiation, and the role of REST on DM remyelination remains to be investigated. Here, we investigated the effects of REST and DNA methylation on DM remyelination and explored the underlying mechanisms. In this study, using a diabetic mouse model, we found that myelin damage preceded neuronal damage and caused cognitive impairment in DM mice. Inhibition of REST by X5050 and DNMT3b by Naomycin A promoted myelin regeneration in the hippocampus and ameliorated cognitive deficits in DM mice. In addition, CpA methylation of the RE-1 locus of the CNTN1 gene was able to increase the binding capacity of REST. We also observed that CNTN1 promotes oligodendrocyte maturation, facilitates the ratio of microglia to pro-regenerative phenotypes as well as enhances the ability of microglia to remove myelin debris. Our findings suggest that REST and DNMT3b expression inhibit CNTN1 expression and exacerbate myelin damage. This mechanism of gene silencing may be associated with DNMT3b-mediated CpA methylation of the REST binding site in the promoter region of the CNTN1 gene. We also identified the role for CNTN1 in promoting oligodendrocyte precursor cell maturation and myelin debris removal during remyelination.
Keywords: CNTN1; DNA methylation; REST; demyelination; diabetes mellitus; oligodendrocyte.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019;157 - PubMed
-
- Grunblatt E., Bartl J., Riederer P. The link between iron, metabolic syndrome, and Alzheimer's disease. J. Neural. Transm. (Vienna) 2011;118:371–379. - PubMed
-
- Biessels G.J., Staekenborg S., Brunner E., Brayne C., Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. - PubMed
-
- Wong R.H., Scholey A., Howe P.R. Assessing premorbid cognitive ability in adults with type 2 diabetes mellitus--a review with implications for future intervention studies. Curr. Diab. Rep. 2014;14:547. - PubMed
-
- Bellia C., Lombardo M., Meloni M., Della-Morte D., Bellia A., Lauro D. Diabetes and cognitive decline. Adv. Clin. Chem. 2022;108:37–71. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

